Mulberrofuran QCAS# 101383-35-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 101383-35-1 | SDF | Download SDF |
| PubChem ID | 5319933 | Appearance | Powder |
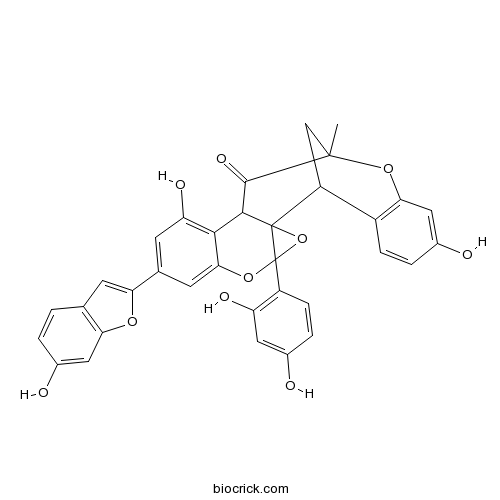
| Formula | C34H24O10 | M.Wt | 592.55 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-(2,4-dihydroxyphenyl)-10,18-dihydroxy-8-(6-hydroxy-1-benzofuran-2-yl)-14-methyl-3,5,15-trioxahexacyclo[12.7.1.02,4.02,12.06,11.016,21]docosa-6,8,10,16(21),17,19-hexaen-13-one | ||
| SMILES | CC12CC(C3=C(O1)C=C(C=C3)O)C45C(C2=O)C6=C(C=C(C=C6OC4(O5)C7=C(C=C(C=C7)O)O)C8=CC9=C(O8)C=C(C=C9)O)O | ||
| Standard InChIKey | MSVXRBNAPJJEDX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C34H24O10/c1-32-14-22(20-6-4-19(37)13-27(20)42-32)33-30(31(32)40)29-24(39)8-16(25-9-15-2-3-18(36)12-26(15)41-25)10-28(29)43-34(33,44-33)21-7-5-17(35)11-23(21)38/h2-13,22,30,35-39H,14H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mulberrofuran Q may protect neuronal cell death against the oxidative stress induced by oxygen-glucose deprivation (OGD). |
| In vitro | Protection of prenylated flavonoids from Mori Cortex Radicis (Moraceae) against nitric oxide-induced cell death in neuroblastoma SH-SY5Y cells.[Pubmed: 22297755]Arch Pharm Res. 2012 Jan;35(1):163-70.
Inhibitory effect of 2-arylbenzofurans from the Mori Cortex Radicis (Moraceae) on oxygen glucose deprivation (OGD)-induced cell death of SH-SY5Y cells.[Pubmed: 21910060]Arch Pharm Res. 2011 Aug;34(8):1373-80.
|
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2009 Feb;34(3):286-90.Study on Diels-Alder type adducts from stem barks of Morus yunanensis.[Pubmed: 19445150]To isolate and identify the Diels-Alder type adducts from stem barks of Morus yunanensis.
|

Mulberrofuran Q Dilution Calculator

Mulberrofuran Q Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6876 mL | 8.4381 mL | 16.8762 mL | 33.7524 mL | 42.1905 mL |
| 5 mM | 0.3375 mL | 1.6876 mL | 3.3752 mL | 6.7505 mL | 8.4381 mL |
| 10 mM | 0.1688 mL | 0.8438 mL | 1.6876 mL | 3.3752 mL | 4.2191 mL |
| 50 mM | 0.0338 mL | 0.1688 mL | 0.3375 mL | 0.675 mL | 0.8438 mL |
| 100 mM | 0.0169 mL | 0.0844 mL | 0.1688 mL | 0.3375 mL | 0.4219 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydroxy-beta-sanshool
Catalog No.:BCN8841
CAS No.:97465-69-5
- Iso-sagittatoside A
Catalog No.:BCN8840
CAS No.:503456-08-4
- Celosin J
Catalog No.:BCN8839
CAS No.:1623405-29-7
- Arjunetin
Catalog No.:BCN8838
CAS No.:31297-79-7
- Quercetin 3-O-neohesperidoside
Catalog No.:BCN8837
CAS No.:32453-36-4
- 6-Hydroxykaempferol 3-O-beta-D-glucoside
Catalog No.:BCN8836
CAS No.:145134-61-8
- 4H-1-Benzopyran-4-one,2,3-dihydro-5-hydroxy-3-[(4-hydroxyphenyl)methyl]-7-methoxy-
Catalog No.:BCN8835
CAS No.:108001-32-7
- Notoginsenoside R4
Catalog No.:BCN8834
CAS No.:87741-77-3
- Hythiemoside B
Catalog No.:BCN8833
CAS No.:853267-90-0
- Randialic acid B
Catalog No.:BCN8832
CAS No.:14021-14-8
- Harmidol hydrochloride
Catalog No.:BCN8831
CAS No.:6028-07-5
- Cassiaside B2
Catalog No.:BCN8830
CAS No.:218155-40-9
- 4'-Methoxyagarotetrol
Catalog No.:BCN8845
CAS No.:123278-01-3
- Sterebin E
Catalog No.:BCN8846
CAS No.:114343-74-7
- Bacopaside N2
Catalog No.:BCN8847
CAS No.:871706-75-1
- Hyperforin
Catalog No.:BCN8848
CAS No.:11079-53-1
- Isorhamnetin 7-O-alpha-L-rhamnoside
Catalog No.:BCN8850
CAS No.:17331-72-5
- 27-O-acetyl-withaferin A
Catalog No.:BCN8852
CAS No.:1214886-35-7
- Neoastilbin
Catalog No.:BCN8853
CAS No.:54081-47-9
- Rebaudioside E
Catalog No.:BCN8854
CAS No.:63279-14-1
- Gardoside
Catalog No.:BCN8855
CAS No.:54835-76-6
- Rhaponticin 6''-O-gallate
Catalog No.:BCN8856
CAS No.:94356-23-7
- Hellebrigenin
Catalog No.:BCN8857
CAS No.:465-90-7
- 3beta-Methoxy-2,3-dihydrowithaferin A
Catalog No.:BCN8859
CAS No.:73365-94-3


