ArjunetinCAS# 31297-79-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 31297-79-7 | SDF | Download SDF |
| PubChem ID | 3052779 | Appearance | White powder |
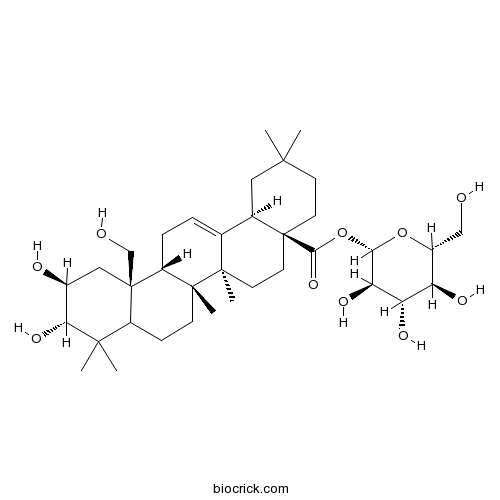
| Formula | C36H58O10 | M.Wt | 650.9 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | 24-Deoxysericoside | ||
| Solubility | Soluble in DMSO and methanol; insoluble in chloroform, n-hexane and water | ||
| Chemical Name | [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] (4aS,6aR,6aS,6bR,10S,11S,12aS,14bR)-10,11-dihydroxy-12a-(hydroxymethyl)-2,2,6a,6b,9,9-hexamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylate | ||
| SMILES | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CC(C(C5(C)C)O)O)CO)C)C2C1)C)C(=O)OC6C(C(C(C(O6)CO)O)O)O)C | ||
| Standard InChIKey | IGWNEOKIHCAVIU-FZFZDMCWSA-N | ||
| Standard InChI | InChI=1S/C36H58O10/c1-31(2)11-13-35(30(44)46-29-27(42)26(41)25(40)22(17-37)45-29)14-12-33(5)19(20(35)15-31)7-8-24-34(33,6)10-9-23-32(3,4)28(43)21(39)16-36(23,24)18-38/h7,20-29,37-43H,8-18H2,1-6H3/t20-,21+,22-,23?,24-,25-,26+,27-,28-,29+,33-,34-,35+,36-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Arjunetin, an insect feeding-deterrent and growth inhibitor, shows antioxidant and anti-inflammatory activities. It demonstrates significant inhibition of(Dipeptidyl peptidase-IV) DPP-IV enzyme, the DPP-IV Inhibitory activity translated into significant cardioprotective effects in the setting of diabetes. |
| Targets | DPP-IV |
| In vitro | Arjunetin from Terminalia arjuna as an insect feeding-deterrent and growth inhibitor.[Pubmed: 15022165 ]Phytother Res. 2004 Feb;18(2):131-4.
Dipeptidyl peptidase IV Inhibitory activity of Terminalia arjuna attributes to its cardioprotective effects in experimental diabetes: In silico, in vitro and in vivo analyses.[Pubmed: 30668318 ]Phytomedicine. 2019 Apr;57:158-165.The marketed synthetic (Dipeptidyl peptidase-IV) DPP-IV Inhibitors are expensive antidiabetic drugs and have been reported to cause unacceptable adverse effects such as pancreatitis, angioedema, thyroid and pancreatic cancers. In this scenario research to develop novel DPP-IV Inhibitors from alternative sources is the need of the hour.
Terminalia arjuna, a medicinal herb with antidiabetic and cardioprotective activities may represent a natural DPP-IV Inhibitor, the DPP-IV Inhibitory activity of which may translate into demonstrable therapeutic benefits in setting of diabetes with cardiovascular co-morbidities.
The study type used for the present study was an experimental (In vitro, In vivo and In silico) design.
|
| Structure Identification | Z Naturforsch C J Biosci. 2017 May 1;72(5-6):203-208.Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential.[Pubmed: 27997356 ]
|

Arjunetin Dilution Calculator

Arjunetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5363 mL | 7.6817 mL | 15.3633 mL | 30.7267 mL | 38.4084 mL |
| 5 mM | 0.3073 mL | 1.5363 mL | 3.0727 mL | 6.1453 mL | 7.6817 mL |
| 10 mM | 0.1536 mL | 0.7682 mL | 1.5363 mL | 3.0727 mL | 3.8408 mL |
| 50 mM | 0.0307 mL | 0.1536 mL | 0.3073 mL | 0.6145 mL | 0.7682 mL |
| 100 mM | 0.0154 mL | 0.0768 mL | 0.1536 mL | 0.3073 mL | 0.3841 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Quercetin 3-O-neohesperidoside
Catalog No.:BCN8837
CAS No.:32453-36-4
- 6-Hydroxykaempferol 3-O-beta-D-glucoside
Catalog No.:BCN8836
CAS No.:145134-61-8
- 4H-1-Benzopyran-4-one,2,3-dihydro-5-hydroxy-3-[(4-hydroxyphenyl)methyl]-7-methoxy-
Catalog No.:BCN8835
CAS No.:108001-32-7
- Notoginsenoside R4
Catalog No.:BCN8834
CAS No.:87741-77-3
- Hythiemoside B
Catalog No.:BCN8833
CAS No.:853267-90-0
- Randialic acid B
Catalog No.:BCN8832
CAS No.:14021-14-8
- Harmidol hydrochloride
Catalog No.:BCN8831
CAS No.:6028-07-5
- Cassiaside B2
Catalog No.:BCN8830
CAS No.:218155-40-9
- Anemarrhenasaponin III
Catalog No.:BCN8829
CAS No.:163047-23-2
- Isorhamnetin 3,7-O-diglucoside
Catalog No.:BCN8828
CAS No.:6758-51-6
- 5'''-O-Feruloyl complanatoside B
Catalog No.:BCN8827
CAS No.:142473-98-1
- 11-Oxomogroside IV
Catalog No.:BCN8826
CAS No.:2096516-32-2
- Celosin J
Catalog No.:BCN8839
CAS No.:1623405-29-7
- Iso-sagittatoside A
Catalog No.:BCN8840
CAS No.:503456-08-4
- Hydroxy-beta-sanshool
Catalog No.:BCN8841
CAS No.:97465-69-5
- Mulberrofuran Q
Catalog No.:BCN8844
CAS No.:101383-35-1
- 4'-Methoxyagarotetrol
Catalog No.:BCN8845
CAS No.:123278-01-3
- Sterebin E
Catalog No.:BCN8846
CAS No.:114343-74-7
- Bacopaside N2
Catalog No.:BCN8847
CAS No.:871706-75-1
- Hyperforin
Catalog No.:BCN8848
CAS No.:11079-53-1
- Isorhamnetin 7-O-alpha-L-rhamnoside
Catalog No.:BCN8850
CAS No.:17331-72-5
- 27-O-acetyl-withaferin A
Catalog No.:BCN8852
CAS No.:1214886-35-7
- Neoastilbin
Catalog No.:BCN8853
CAS No.:54081-47-9
- Rebaudioside E
Catalog No.:BCN8854
CAS No.:63279-14-1
Dipeptidyl peptidase IV Inhibitory activity of Terminalia arjuna attributes to its cardioprotective effects in experimental diabetes: In silico, in vitro and in vivo analyses.[Pubmed:30668318]
Phytomedicine. 2019 Apr;57:158-165.
BACKGROUND: The marketed synthetic (Dipeptidyl peptidase-IV) DPP-IV Inhibitors are expensive antidiabetic drugs and have been reported to cause unacceptable adverse effects such as pancreatitis, angioedema, thyroid and pancreatic cancers. In this scenario research to develop novel DPP-IV Inhibitors from alternative sources is the need of the hour. HYPOTHESIS/PURPOSE: Terminalia arjuna, a medicinal herb with antidiabetic and cardioprotective activities may represent a natural DPP-IV Inhibitor, the DPP-IV Inhibitory activity of which may translate into demonstrable therapeutic benefits in setting of diabetes with cardiovascular co-morbidities. STUDY DESIGN: The study type used for the present study was an experimental (In vitro, In vivo and In silico) design. METHOD: The DPP-IV Inhibitory, antidiabetic and cardioprotective effects of Terminalia arjuna was evaluated in the experimental model of myocardial infarction co-existing with diabetes. To determine the active principle of Terminalia arjuna responsible for DPP-IV Inhibitory activity, the crystal structure of DPP-IV was considered as receptor which was docked against Arjunetin, Arjungenin, Arjunic acid, Arjunone, Ellagic acid, Gallic acid, Sitagliptin and Vildagliptin. The binding sites as well as affinity of various active ingredients of Terminalia arjuna for DPP- IV enzyme was elucidated using in silico studies and compared to Vildagliptin. RESULTS: Terminalia arjuna demonstrated significant DPP-IV Inhibitory, antidiabetic (significant reduction in HbA1C) and cardioprotective effects (restoration of myocardial CPK-MB) in the experimental model of myocardial infarction co-existing with diabetes. The cardioprotective efficacy correlated to its DPP-IV Inhibitory activity. The active ingredients of Terminalia arjuna (Arjunetin, Arjungenin, Arjunic Acid Arjunone, Ellagic acid and Gallic acid) demonstrated significant inhibition of DPP-IV enzyme. Arjunic acid and Arjunone prefers the active site pocket of DPP-IV enzyme. Compounds like Arjunetin and Vildagliptin prefers to bind near the interface region of the DPP-IV as their biological active forms are homodimer. Sitagliptin binds near the alpha/beta hydrolase domain. CONCLUSION: The DPP-IV Inhibitory activity of Terminalia arjuna was found to be comparable to Vildagliptin. The DPP-IV Inhibitory activity translated into significant cardioprotective effects in the setting of diabetes. The active ingredient of Terminalia arjuna; Arjunetin, Arjungenin, Ellagic acid and Arjunic acid showed superior DPP-IV Inhibitory activity as compared to synthetic DPP-IV inhibitors (Sitagliptin and Vildagliptin) based on results of docking studies.
Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential.[Pubmed:27997356]
Z Naturforsch C J Biosci. 2017 May 1;72(5-6):203-208.
Termiglaucescin (1), a new triterpene glucoside, has been isolated from the ethyl acetate extract of the root bark of Terminalia glaucescens Planch. ex Benth, together with 11 known compounds, beta-D-glucopyranosyl 2alpha,3beta,6beta-trihydroxy-23-galloylolean-12-en-28-oate (2), arjunglucoside I (3), sericoside (4), arjungenin (5), sericic acid (6), Arjunetin (7), chebuloside II (8), 3,3',4-tri-O-methylelagic acid (9), 3,3'-di-O-methylelagic acid (10), beta-sitosterol (11) and stigmasterol (12). Compounds 2, 3, 7, 8 and 9 are reported from the plant for the first time. The structures of the isolated compounds were characterized by spectroscopic data interpretations, especially 1D and 2D NMR. The triterpenic isolates showed potent antioxidant and anti-inflammatory activities.
Evaluation of herb-drug interaction of a polyherbal Ayurvedic formulation through high throughput cytochrome P450 enzyme inhibition assay.[Pubmed:27457692]
J Ethnopharmacol. 2017 Feb 2;197:165-172.
ETHNOPHARMACOLOGICAL RELEVANCE: Arishtas are Ayurvedic formulation made with decoction of herbs. Arjunarishta formulation is being used in Ayurveda for cardio-protective activity. Ashwagandharishta formulation possesses antioxidant, anti-atherosclerotic and anti-stress properties. Ridayarishta, a novel empirical formulation was prepared using combination of selected ingredients from these two formulations to support healthy heart functions and to reduce stress. AIM OF THE STUDY: Aim of the Study was to investigate herb-drug interaction (HDI) of Ridayarishta formulation through human hepatic cytochrome P450 (CYP450) enzyme inhibition assay. MATERIALS AND METHODS: Ridayarishta formulation was phyto-chemically standardized against arjunolic acid, Arjunetin, berberine, piperine, resveratrol and withaferin-A using high performance thin layer chromatography (HPTLC) analysis. The formulation was standardized with respect to ethanol by gas chromatographic (GC) analysis. HDI was evaluated with Ridayarishta formulation and amlodipine besilate, atenolol, atorvastatin, metformin, glipizide glimepiride cocktail using high throughput CYP450 enzyme inhibition assay; against CYP1A2, 2C19, 2D6 and 3A4 isozymes. RESULTS: Contents of arjunolic acid, Arjunetin, berberine, piperine, resveratrol and withaferin-A in Ridayarishta formulation were found to be 1.76+/-0.12, 1.51+/-0.09, 1.85+/-0.05, 3.2+/-0.12, 1.21+/-0.08, and 2.16+/-0.09ppm, respectively. Quantity of ethanol in Ridayarishta was found to be 7.95+/-0.023% (V/V). Ridayarishta showed significantly higher (P<0.001) IC50 value against CYP1A2 (IC50-13.80+/-1.96microg/mL), 2C19 (IC50-14.343+/-2.28microg/mL), 2D6 (IC50-0.897+/-0.28microg/mL) and 3A4 (IC50-32.057+/-2.51microg/mL) compared to positive controls such as furafylline, tranylcypromine, quinidine and ketoconazole respectively. Cocktail of herbal formulation and cardio protective, antihypertensive, anti-diabetic drugs showed significantly (P<0.001and P<0.01) less or negligible HDI. CONCLUSION: Ridayarishta formulation alone and cocktail with amlodipine besilate, atenolol, atorvastatin, metformin, glipizide, glimepiride had negligible or insignificant effect on CYP450 inhibition. It may be concluded that consumption of Ridayarishta along with selective cardio protective, antihypertensive and anti-diabetic conventional medicine is safe with negligible or without any significant CYP450 (CYP1A2, 2C19, 2D6 and 3A4) inhibition mediated HDI.
In Vitro CYP2D Inhibitory Effect and Influence on Pharmacokinetics and Pharmacodynamic Parameters of Metoprolol Succinate by Terminalia arjuna in Rats.[Pubmed:26891872]
Drug Metab Lett. 2016;10(2):124-35.
BACKGROUND: Terminalia arjuna Wight & Arn. (Combretaceae) is a tree having an extensive medicinal potential in cardiovascular disorders. T. arjuna bark extract has been reported to play a significant role as a cardiac stimulant for its beneficial effects in angina. Herb - drug interactions (HDI) are one of the most important clinical concerns in the concomitant consumption of herbs and prescription drugs. Our study was to investigate the in vitro CYP2D inhibition potential of Terminalia arjuna (T. arjuna) extracts in rat liver microsomes and to study the influence of aqueous bark extract of T. arjuna on the oral pharmacokinetics and pharmacodynamics of metoprolol succinate in rats. METHODS: The CYP2D inhibition potential of herbal extracts of T. arjuna was investigated in rat liver microsomes. Pharmacokinetic-pharmacodynamic interaction of aqueous extract of T. arjuna with metoprolol succinate was investigated in rats. RESULTS: The ethyl acetate, alcoholic & aqueous bark extracts of T. arjuna showed potent reversible non-competitive inhibition CYP2D enzyme in rat liver microsomes with IC50 values less than 40 mug/mL. Arjunic acid, Arjunetin and arjungenin did not show significant inhibition of CYP2D enzyme in rat liver microsomes. Pharmacokinetic studies showed that aqueous bark extract of T. arjuna led to a significant reduction (P < 0.05) in AUC0-24h and Cmax of metoprolol succinate in rats, when co-administered. Pharmacodynamic studies reveal a significant reduction in therapeutic activity of metoprolol succinate on co-administration with aqueous bark extract of T. arjuna. CONCLUSION: Based on our in vitro and in vivo findings and until further clinical drug interaction experiments are conducted, the co-administration of drugs, especially those primarily cleared via CYP2D catalyzed metabolism, with T. arjuna extracts should be done with caution.
[Chemical constituents from medical and edible plants of Rosa roxburghii].[Pubmed:28868863]
Zhongguo Zhong Yao Za Zhi. 2016 Feb;41(3):451-455.
Rosa roxburghii, a kind of the medical and edible plants belonging to the Rosaceae family, is widely distributed in the southwest districts of China, especially Guizhou province. Now, by reason of the extensive bioactivities, the plant is widely used in the field of food, health product, drug, and so on. In the course of our continuing search for the bioactive constituents, thirteen compounds were isolated from R. roxburghii, and their structures were determined on the basis of physicochemical property, spectroscopic data and comparison with the literatures, as 2-oxo pomolic acid(1), 1beta-hydroxyeuscaphic acid(2), euscaphic acid(3), arjunic acid(4), tormentic acid(5), kaiiichigeside F1(6), rosamultin(7), Arjunetin(8), 2a, 3a, 19a-trihydroxy-olean-12-en-28-oic acid 28-O-beta-D-glucopyranoside(9), 2alpha, 3alpha, 19alpha, 24-tetrahydroxyolean-12-en-28-oic-acid 28-O-beta-D-glucopyranosyl ester(10), pyrogallic acid (11), daucosterol(12), and 1, 2-decanediol(13). Compounds 9 and 10 were firstly obtained from Rosaceae family, and compounds 1,4,5,9-11,13 were isolated from this plant for the first time.
In vitro modulatory effects of Terminalia arjuna, arjunic acid, arjunetin and arjungenin on CYP3A4, CYP2D6 and CYP2C9 enzyme activity in human liver microsomes.[Pubmed:28962416]
Toxicol Rep. 2015 Feb 17;2:806-816.
Terminalia arjuna is a tree having an extensive medicinal potential in cardiovascular disorders. Triterpenoids are mainly responsible for cardiovascular properties. Alcoholic and aqueous bark extracts of T. arjuna, arjunic acid, Arjunetin and arjungenin were evaluated for their potential to inhibit CYP3A4, CYP2D6 and CYP2C9 enzymes in human liver microsomes. We have demonstrated that alcoholic and aqueous bark extract of T. arjuna showed potent inhibition of all three enzymes in human liver microsomes with IC50 values less than 50 mug/mL. Arjunic acid, Arjunetin and arjungenin did not show significant inhibition of CYP enzymes in human liver microsomes. Enzyme kinetics studies suggested that the extracts of arjuna showed reversible non-competitive inhibition of all the three enzymes in human liver microsomes. Our findings suggest strongly that arjuna extracts significantly inhibit the activity of CYP3A4, CYP2D6 and CYP2C9 enzymes, which is likely to cause clinically significant drug-drug interactions mediated via inhibition of the major CYP isozymes.
Simultaneous determination and characterization of tannins and triterpene saponins from the fruits of various species of Terminalia and Phyllantus emblica using a UHPLC-UV-MS method: application to triphala.[Pubmed:23299756]
Planta Med. 2013 Jan;79(2):181-8.
Terminalia species are a rich source of tannins. Many preparations of these species are used in traditional medicine and have many different ethnobotanical applications. A simple UHPLC method was developed for the simultaneous analysis of such hydrolysable tannins and triterpene saponins from the fruit rinds of different species of Terminalia (T. chebula, T. arjuna, T. bellirica) and Phyllantus emblica. A separation by LC was achieved using a reversed-phase column and a water/acetonitrile mobile phase, both containing formic acid, using a gradient system and a temperature of 40 degrees C. Eight hydrolysable tannins (gallic acid, gallic acid methyl ester, corilagin, chebulagic acid, 1,2,3,6-tetra-O-galloyl-beta-D-glucose, ellagic acid, chebulinic acid, and 1,2,3,4,6-penta-O-galloyl-beta-D-glucose) and six triterpene saponins (arjunglucoside-I, arjunglucoside-III, chebuloside II, bellericoside, Arjunetin, and arjunglucoside-II) could be separated within 20 minutes. The wavelength used for detection with the diode array detector was 254 and 275 nm for tannins and 205 nm for triterpene saponins. The method was validated for linearity, repeatability, limits of detection, and limits of quantification. The developed method is economical, fast, and especially suitable for quality control analysis of tannins and triterpene saponins in various plant samples and commercial products of Terminalia.
[Triterpene saponins from Adinandra nitida].[Pubmed:18717338]
Yao Xue Xue Bao. 2008 May;43(5):504-8.
To investigate the chemical constituents of the leaves of Adinandra nitida, several column chromatography methods were used to isolate the chemical constituents of this plant. The structures were elucidated on the basis of spectral data. Six compounds were isolated and identified as 2alpha, 3alpha, 19alpha-trihydroxy-olean-12-en-28-oic acid-28-O-beta-D-glucopyranoside (1), Arjunetin (2), sericoside (3), glucosyl tormentate (4), nigaichigoside F1 (5) and arjunglucoside I (6), separately. Compound 1 is a new compound, and compounds 2 -6 were isolated from A. nitida for the first time.
Cytotoxic agents from Terminalia arjuna.[Pubmed:18008199]
Planta Med. 2007 Nov;73(14):1486-90.
Although a number of chemicals have been isolated from Terminalia arjuna, only a few have been evaluated for their biological significance. As a part of our drug discovery programme for cytotoxic agents from Indian medicinal plants, four novel cytotoxic agents arjunic acid (1), arjungenin (2), Arjunetin (3) and arjunoglucoside I (4) were isolated from the bark of T. ARJUNA. Out of the four compounds, arjunic acid (1) was significantly active against the human oral (KB), ovarian (PA 1) and liver (HepG-2 & WRL-68) cancer cell lines. Further, the most active compound arjunic acid was converted into seven semi-synthetic ester derivatives 5 - 11. 2-O-Palmitoyl arjunic acid (6) showed two times more activity, while 2, 3-di-O-acetyl-, 2-O-p-anisoyl-, 2, 3-di-O-benzoyl- and 2, 3-di-O-p-nitrobenzoyl arjunic acid (7 - 10) showed 1.7 - 2.3 times less activity than the cytotoxic drug vinblastine against the liver cancer cell lines HepG-2 and WRL-68 respectively.
Effect of oleanane triterpenoids from Terminalia arjuna--a cardioprotective drug on the process of respiratory oxyburst.[Pubmed:15957375]
Phytomedicine. 2005 May;12(5):391-3.
The oleanane triterpenes arjunic acid, arjungenin and their glucosides, Arjunetin and arjunglucoside II, were isolated from the bark of Terminalia arjuna. Arjungenin and its glucoside exhibited a moderate free radical scavenging activity while all the compounds showed no effect on the superoxide release from PMN cells. Further arjungenin also exhibited greater inhibitory action on the hypochlorous acid production from human neutrophils.
Arjunetin from Terminalia arjuna as an insect feeding-deterrent and growth inhibitor.[Pubmed:15022165]
Phytother Res. 2004 Feb;18(2):131-4.
Crude ethanolic extract of the stem bark of Terminalia arjuna (Combretaceae) and its three compounds namely arjunic acid, arjungenin and Arjunetin were evaluated for antifeedant, growth inhibitory and oviposition-deterrent activities against a lepidopterous insect Spilarctia obliqua. The compound Arjunetin showed highest growth inhibitory and feeding-deterrent properties with a growth inhibition (GI(50)) and feeding-inhibition (FD(50)) of 188.5 and 287.1 micro g/g diet respectively. Oviposition bioassays indicated no oviposition-deterrence in any of the compounds tested. The structure-activity relationship study indicated the importance of a glycosidation linkage in Arjunetin.
Quantitative determination of oleane derivatives in Terminalia arjuna by high performance thin layer chromatography.[Pubmed:12184173]
Phytochem Anal. 2002 Jul-Aug;13(4):207-10.
A simple, precise and rapid high performance thin layer chromatographic method has been developed for the simultaneous quantitative determination of five oleane derivatives, namely, arjunic acid, arjunolic acid, arjungenin, Arjunetin and arjunglucoside I from stem bark extract of Terminalia arjuna. The isolation and separation of these compounds was carried out on 60F254 layers eluted with chloroform:methanol (90:10), and the analytes were visualised through colour development with vanillin in concentrated sulphuric acid:ethanol. Scanning and quantification of the spots at 640 nm showed good recoveries in the range 96.40-101.7%.
Efficacy of Terminalia arjuna in chronic stable angina: a double-blind, placebo-controlled, crossover study comparing Terminalia arjuna with isosorbide mononitrate.[Pubmed:12086380]
Indian Heart J. 2002 Mar-Apr;54(2):170-5.
BACKGROUND: Terminalia arjuna, an Indian medicinal plant, has been reported to have beneficial effects in patients with ischemic heart disease in a number of small, open studies. The need for a double-blind, randomized, placebo-controlled study with adequate sample size has long been felt. The bark extract (IPC-53) contains acids (arjunic acid, terminic acid), glycosides (Arjunetin arjunosides I-IV), strong antioxidants (flavones, tannins, oligomeric proanthocyanidins), minerals. etc. and exhibits antifailure and anti-ischemic properties. METHODS AND RESULTS: Fifty-eight males with chronic stable angina (NYHA class II-III) with evidence of provocable ischemia on treadmill exercise test received Terminalia arjuna (500 mg 8 hourly), isosorbide mononitrate (40 mg/daily) or a matching placebo for one week each, separated by a wash-out period of at least three days in a randomized, double-blind, crossover design. They underwent clinical, biochemical and treadmill exercise evaluation at the end of each therapy which were compared during the three therapy periods. Terminalia arjuna therapy was associated with significant decrease in the frequency of angina and need for isosorbide dinitrate (5.69+/-6.91 mg/week v. 18.22+/-9.29 mg/week during placebo therapy, p<0.005). The treadmill exercise test parameters improved significantly during therapy with Terminalia arjuna compared to those with placebo. The total duration of exercise increased (6.14+/-2.51 min v. 4.76+/-2.38 min, p<0.005), maximal ST depression during the longest equivalent stages of submaximal exercise decreased (1.41+/-0.55 mm v. 2.21+/-0.56 mm, p<0.005), time to recovery decreased (6.49+/-2.37 min v. 9.27+/-3.39 min, p<0.005) and higher double products were achieved (25.75+/-4.81x10(3) v. 23.11+/-4.83x10(3), p<0.005) during Terminalia arjuna therapy. Similar improvements in clinical and treadmill exercise test parameters were observed with isosorbide mononitrate compared to placebo therapy. No significant differences were observed in clinical or treadmill exercise test parameters when Terminalia arjuna and isosorbide mononitrate therapies were compared. No significant untoward effects were reported during Terminalia arjuna therapy. CONCLUSIONS: Terminalia arjuna bark extract, 500 mg 8 hourly, given to patients with stable angina with provocable ischemia on treadmill exercise, led to improvement in clinical and treadmill exercise parameters as compared to placebo therapy. These benefits were similar to those observed with isosorbide mononitrate (40 mg/day) therapy and the extract was well tolerated. Limitations of this study include applicability of the results to only men with chronic stable angina but not necessarily to women, as they were not studied.
RP-LC determination of oleane derivatives in Terminalia arjuna.[Pubmed:12008123]
J Pharm Biomed Anal. 2002 May 15;28(3-4):447-52.
A rapid sensitive and reproductive reversed phase high performance liquid chromatographic method with photo diode arrray detection is described for the simultaneous quantification of major oleane derivatives: arjunic acid (4), arjunolic acid (3), arjungenin (2) and Arjunetin (1) in Terminalia arjuna extract. The method involves the use of a Waters Spherisorb S10 ODS2 column (250 x 4.6 mm, I.D., 10 microm) and binary gradient mobile phase profile. The various other aspects of analysis viz. Extraction efficiency, peak purity and similarity were validated using a photo diode array detector.
Quadranosides VI-XI, six new triterpene glucosides from the seeds of Combretum quadrangulare.[Pubmed:10959573]
Chem Pharm Bull (Tokyo). 2000 Aug;48(8):1114-20.
Six new triterpene glucosides, quadranosides VI-XI (1-6), belonging to three different [ursane- (1-4), oleanane- (5) and lupane-type (6)] triterpene classes, have been isolated from a MeOH extract of the seeds of Combretum quadrangulare KURZ (Combretaceae), together with nine known compounds, rosamutin (7), 28-O-beta-D-glucopyranosyl-6beta,23-dihydroxytormentic acid (8), Arjunetin (9), arjunglucoside II (10), combreglucoside (11), chebuloside II (12), vitexin (13), (+)-catechin (14) and (-)-epigallocatechin (15). The structures of these compounds were elucidated by spectroscopic analysis.


