Nymphaea candida
Nymphaea candida
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Nymphaea candida
- Cat.No. Product Name CAS Number COA
-
BCN5533
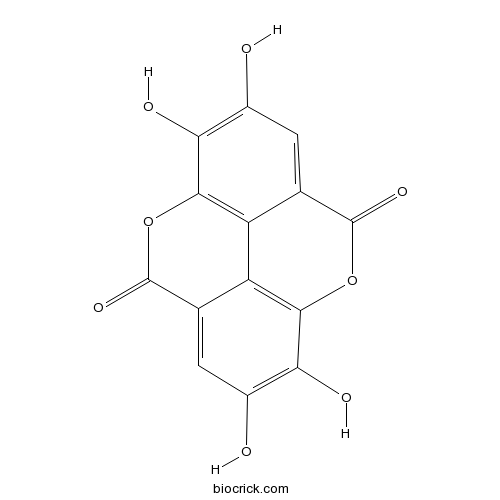
Ellagic acid476-66-4
Instructions
-
BCN2402
Geraniin60976-49-0
Instructions

Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and D-Galactosamine-Induced Liver Injury in Mice.[Pubmed: 28282879]
Nymphaea candida was used to treat hepatitis in Ugyhur medicine, and nicotiflorin (kaempferol 3-O-β-rutinoside) is the main characteristic component in this plant [...].
Antioxidant and preventive effects of extract from nymphaea Candida flower on in vitro immunological liver injury of rat primary hepatocyte cultures.[Pubmed: 19196740]
Nymphaea candida is traditional Uighur medicine that is commonly used to treat head pains, cough, hepatitis and hypertension in Xinjiang of China. In this article, the extract of N. candida was measured for antioxidant activity, using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals scavenging assay and reducing power determination, and compared with those of the positive controls of butylated hydroxytoluene (BHT) and gallic acid (GA). The active extract was further purified by liquid-liquid partition to afford four fractions, of which the ethyl acetate-soluble (EA) fraction (NCE) exhibited the strongest antioxidant capacity with IC(50) value of 12.6 μg/mL for DPPH. Thirteen phenolic compounds were isolated from this fraction, and they all showed significant antioxidant activities in DPPH model system. Furthermore, NCE showed potent antioxidant capacity with IC(50) value of 59.32 μg/mL, 24.48 μg/mL and 86.85 μg/mL, for O(2) (-), ·OH and H(2)O(2) radicals, respectively. Moreover, NCE on BCG plus LPS-induced immunological liver injury was evaluated using primary cultured rat hepatocytes. NCE produced significant hepatoprotective effects as evidenced by decreased supernatant enzyme activities (AST-aspartate transaminase, P < .01; ALT-alanine transferase, P < .01) and nitric oxide (NO, P < .01) production. These results revealed the in vitro antioxidant and hepatoprotective activities of NCE against immunological liver injury. Further investigations are necessary to verify these activities in vivo.
A new flavonol glycoside and activity of compounds from the flower of Nymphaea candida.[Pubmed: 17613618]
A new compound, kaempferol 3-O-(2''-O-galloylrutinoside) (1), was isolated from the white flower of Nymphaea candida, together with nine known flavonol glycosides, kaempferol (2), kaempferol 3-O-beta-D-glucopyranoside (3), kaempferol 3-O-alpha-l-rhamnopyranoside (4), kaempferol 3-O-alpha-l-rhamnopyranosylglucopyranoside (5), kaempferol 7-O-beta-D-glucopyranoside 3-(O-alpha-l-rhamnopyranosylglucopyranoside) (6), quercetin (7), quercetin 3-O-beta-D-xylopyranoside (8), myricetin (9), myricetin 3'-O-beta-D-xylopyranoside (10). The structure of 1 was established on the basis of the analysis of its 1D and 2D NMR spectral data. Compounds 1-7 and 9 exhibited moderate to significant antioxidant activities, which were evaluated by measurement of low-density lipoprotein (LDL) and malondialdehyde (MDA) levels in vitro. Compounds 1, 3, 4, 6 and 9 exhibited promising neuroprotective effects on ischemic injury model of cultured rat cortical neurons treated with sodium dithionite in glucose-free medium. Furthermore, compounds 1, 5, and 9 had distinct cytotoxicity to adrenal gland pheochromocytoma, PC12 cells, being treated by the same way.


