Pilea cavaleriei
Pilea cavaleriei
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Pilea cavaleriei
- Cat.No. Product Name CAS Number COA
-
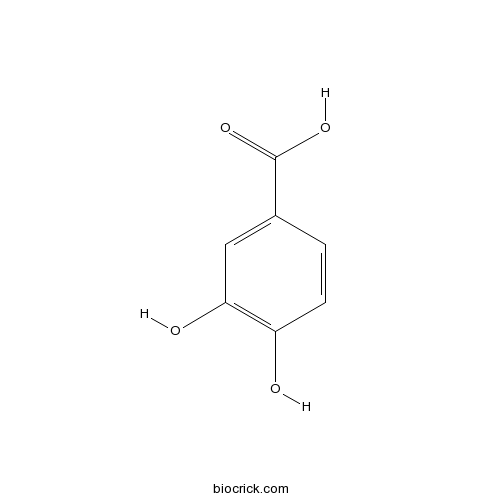
BCN1206
Palmitic acid57-10-3
Instructions

-
BCN4537
3,4-Dihydroxybenzoic acid99-50-3
Instructions
Two new p-coumaroylated sesquiterpenoids from Pilea cavaleriei.[Pubmed: 28480740]
A new p-coumaroylated santalane-type sesquiterpenoid, 8-p-coumaroyl-α-santalene (1), a new p-coumaroylated oplopanane-type sesquiterpenoid, 8-β-p-coumaroyl-oplopanone (2), and three known p-coumaroylated humulene-type sesquiterpenoids (3-5) were isolated from the ethanol extract of the whole herbs of Pilea cavaleriei. Their structures were elucidated based on the combination of 1D and 2D NMR and HRMS methods. Compound 2 was found to show anti-tuberculosis activity with MIC of 16 μg/ml.
Chemical constituents from the whole plants of Pilea cavaleriei Levl subsp. cavaleriei.[Pubmed: 28408268]
Three new sesquiterpene glycosides (1-3), three new glycerol glycosides (4-6), two new alkaloids (7-8), together with seven known compounds (9-15) all of which were isolated from the genus Pilea for the first time, were isolated from the whole plants of Pilea cavaleriei Levl subsp. cavaleriei. Their structures were determined by extensive spectroscopic techniques and chemical methods. The cytotoxic activity of the isolated compounds was evaluated against four cancer cell lines, and none of the tested compounds caused a significant reduction of the cell number.
New phenolic glycosides from Pilea cavaleriei.[Pubmed: 24911100]
Five new phenolic glycosides, 2-hydroxy-(2'E)-prenyl benzoate-2,4'-di-O-β-D-glucopyranoside (1), 2-hydroxy-(2'E)-prenyl benzoate-2-O-α-L-arabinopyranosyl-(1 → 6)-β-D-glucopyranoside (2), 4-methylphenol-1-O-α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranoside (3), 4-methylphenol-1-O-α-L-arabinopyranosyl-(1 → 6)-β-D-glucopyranoside (4), and 3,5-dimethoxyphenol-1-O-β-D-apiofuranosyl-(1 → 2)-β-D-glucopyranoside (5), together with six known glycosides (6-11), were isolated from the n-BuOH fraction of the EtOH extract of Pilea cavaleriei Levl subsp. cavaleriei. Their structures were elucidated by extensive spectroscopic analysis, including 1D and 2D NMR spectroscopy as well as HR-ESI-MS, and chemical evidences. All these compounds were isolated from the genus Pilea for the first time.
Humulane-type sesquiterpenoids from Pilea cavaleriei subsp. crenata.[Pubmed: 23764729]
Nine new, uncommon humulane-type sesquiterpenoids (1, 2, 4, 6-11), together with two known derivatives, were isolated from extracts of the plant Pilea cavaleriei subsp. crenata. The structures of these compounds were fully elucidated by extensive analyses of spectroscopic data (MS, 1D- and 2D-NMR), use of the Mosher method, and by X-ray crystallographic analysis, in combination with chemical conversions. An ene reaction was discovered during the chemical transformations, which might provide an explanation for the wide distribution of the allylic hydroperoxide group in natural products.
[Chemical constituents of Pilea cavaleriei subsp. cavaleriei].[Pubmed: 23236755]
To investigate chemical constituents from folk herb Pilea cavaleriei subsp. cavaleriei.
A new triterpenoid and a new glycoside from Pilea cavaleriei.[Pubmed: 22924790]
A new triterpenoid, 11α,12α-epoxy-3β-hydroxy-24-nor-olean-4(23)-en-28,13β-olide (1), and a new glycoside, benzyl 2-O-β-d-apiofuranosyl-(1 → 2)-β-d-glucopyranosyl benzoate (2), together with eight known triterpenoids (3-10), were isolated from Pilea cavaleriei subsp. cavaleriei. Their structures were established on the basis of spectroscopic analysis including HR-ESI-MS, 1D NMR, and 2D NMR techniques. All compounds showed no anti-hepatitis C virus activity.
Sesquiterpenoids from Pilea cavaleriei subsp. crenata.[Pubmed: 19700322]
Three new humulane-type sesquiterpenes, 8-O-(p-coumaroyl)-5beta-hydroperoxy-1(10)E,4(15)-humuladien-8alpha-ol (1), 8-O-(3-nitro-p-coumaroyl)-1(10)E,4(15)-humuladien-5beta,8alpha-diol (2) and 8-O-(p-coumaroyl)-1(10)E,4(5)E-humuladien-8-ol (3), and a new copaborneol derivative, 1-O-p-coumaroyl-copaborneol (4), have been isolated from the methanol extract of Pileacavaleriei Lévl. subsp. crenata C. J. Chen. Their structures were elucidated using spectroscopic methods. Cytotoxic and antimicrobial activities of the isolated compounds were evaluated.


