Piper kadsura
Piper kadsura
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Piper kadsura
- Cat.No. Product Name CAS Number COA
-
BCC9008
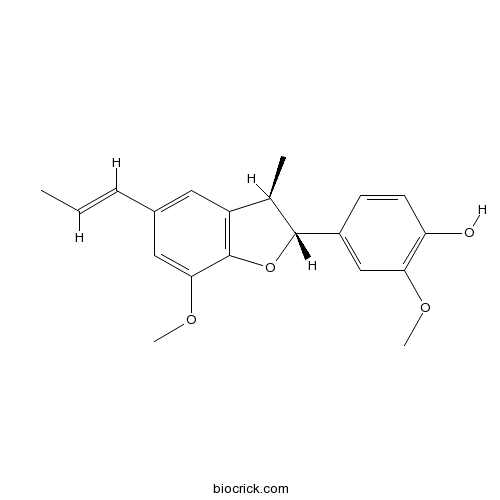
(+)-Licarin A51020-86-1
Instructions
Rapid purification of diastereoisomers from Piper kadsura using supercritical fluid chromatography with chiral stationary phases.[Pubmed: 28641835]
Supercritical fluid chromatography (SFC) with chiral stationary phases (CSPs) is an advanced solution for the separation of achiral compounds in Piper kadsura. Analogues and stereoisomers are abundant in natural products, but there are obstacles in separation using conventional method. In this paper, four lignan diastereoisomers, (-)-Galbelgin, (-)-Ganschisandrin, Galgravin and (-)-Veraguensin, from Piper kadsura were separated and purified by chiral SFC. Purification strategy was designed, considering of the compound enrichment, sample purity and purification throughput. Two-step achiral purification method on chiral preparative columns with stacked automated injections was developed. Unconventional mobile phase modifier dichloromethane (DCM) was applied to improve the sample solubility. Four diastereoisomers was prepared at the respective weight of 103.1mg, 10.0mg, 152.3mg and 178.6mg from 710mg extract with the purity of greater than 98%.
Suitable DNA Barcoding for Identification and Supervision of Piper kadsura in Chinese Medicine Markets.[Pubmed: 27626403]
Piper kadsura is a vine-like medicinal plant which is widely used in clinical treatment. However, P. kadsura is often substituted by other materials in the markets, thereby causing health risks. In this study, 38 P. kadsura samples and eight sequences from GenBank, including a closely-related species and common adulterants were collected. This study aimed to identify an effective DNA barcode from four popular DNA loci for P. kadsura authentication. The success rates of PCR amplification, sequencing, and sequence acquisition of matK were 10.5%, 75%, and 7.9%, respectively; for rbcL they were 89.5%, 8.8%, and 7.9%, respectively; ITS2 rates were 86.8%, 3.0%, and 2.6%, respectively, while for psbA-trnH they were all 100%, which is much higher than for the other three loci. The sequences were aligned using Muscle, genetic distances were computed using MEGA 5.2.2, and barcoding gap was performed using TAXON DNA. Phylogenetic analysis showed that psbA-trnH could clearly distinguish P. kadsura from its closely related species and the common adulterant. psbA-trnH was then used to evaluate the fake proportions of P. kadsura. Results showed that 18.4% of P. kadsura samples were fake, indicating that adulterant species exist in the Chinese markets. Two-dimensional DNA barcoding imaging of P. kadsura was conducted, which was beneficial to the management of P. kadsura. We conclude that the psbA-trnH region is a powerful tool for P. kadsura identification and supervision in the current medicine markets.
The complete plastid genome of Piper kadsura (Piperaceae), an East Asian woody vine.[Pubmed: 26260180]
We sequenced the complete plastid genome (plastome) for Piper kadsura, a woody vine endemic to East Asia. This species is part of the largest genus within Piperaceae and its genome is almost identical to its congener P. cenocladum. The plastome for P. kadsura comprises 131 genes, including four unique rRNAs, 30 tRNAs, and 79 protein-coding genes. It retains ycf1 as an intact open reading frame. Our phylogenetic analysis demonstrated the monophyly of the Piper genus. The additional plastome sequence found in this evolutionarily and economically important genus will be a valuable, fundamental tool for future studies of phylogenetic relationships among basal angiosperms, and will provide a useful resource for molecular breeding programs.
[Identification of some Piper crude drugs based on Fourier transform infrared spectrometry].[Pubmed: 25532337]
The common peak ratio and variant peak ratio were calculated by FTIR spectroscopy of seven medicinal plants of Piper. The dual index sequence of common peak ratio and variant peak ratio was established, which showed the sibship of the medicinal plants. The common peak ratio of Piper kadsura (Choisy) Ohwi, Piper wallichii (Miq.) Hand.-Mazz. Piper laetispicum (C. DC.) was greater than 77%, and the variant peak ratio was less than 30%. The results showed the near sibship between the three drugs. The common peak ratio of Piper kadsura (Choisy) Ohwi, Piper nigrum L. and Piper boehmeriae folium Wall (Miq.) C. DC. Var. tonkinense (C. DC.) was about 61% which showed the farther sibship. The common peak ratio of Piper kadsura (Choisy) Ohwi and Piper betle (Linn.) was only 44%, which showed the farthest sibship. Piper kadsura (choisy) Ohwi and its adulterants, such as Piper wallichii (Miq.) Hand. -Mazz., Piper boehmeriaefolium Wall (Miq.) C. DC. Var. tonkinense C. DC. , Piper laetispicum C. DC., Piper nigrum L., could be identified by comparing their second order derivative IR spectrum of the samples. FTIR technique is a non-destructive analysis method which provides information of functional group, type and hydrogen bond without complex pretreatment procedures such as extraction and separatioin. FTIR method has some characteristics such as rapid and simple analysis procedure, good reproducibility, non-destructive testing, few amount of required sample and low cost and is environment-friendly. The method solved the problems of limit in resource of Piper kadsura (Choisy) Ohwi, many fakes and difficulties in identification, and brought the security for the clinical medication. FTIR provides a new method for identification of Piper kadsura (choisy) Ohwi and its fakes and meets the requirement for comprehensive analy sis and global analysis of traditional Chinese medicine.
The chemical constituents of Piper kadsura and their cytotoxic and anti-neuroinflammtaory activities.[Pubmed: 20687793]
The n-hexane and CHCl₃ soluble fractions of the MeOH extract of the aerial parts of Piper kadsura were found to potently inhibit nitric oxide (NO) production in LPS-activated BV-2 cells, a microglial cell line. From the active fractions, a new stereoisomer of guaiane sesquiterpene, 1α,5β-guai-4(15)-ene-6β,10β-diol, kadsuguain A (1) and a new cyclohexadienone, kadsuketanone A (2), together with twelve known compounds (3-14) were isolated. The structures of these compounds were elucidated by extensive NMR spectral studies. The absolute configuration of 2 was determined by circular dichroism (CD) spectra. Compounds 2, 6, and 11-14 significantly inhibited both nitric oxide (NO) and prostaglandin E₂ (PGE₂) production in the LPS-activated microglia cells. In addition, compounds 4, 6, and 11-14 exhibited cytotoxicity against the A549, SK-OV-3, SK-MEL-2, and HCT15 human tumour cells.
Neolignans from Piper kadsura and their anti-neuroinflammatory activity.[Pubmed: 19900811]
Bioassay-guided column chromatographic separation of the methanolic extract of dried aerial parts of Piper kadsura (Piperaceae) led to the isolation of a new neolignan, piperkadsin C (1), together with eight known neolignans (2-9). The structures of the isolated compounds were elucidated by combined spectroscopic methods. The anti-neuroinflammatory activities of these compounds were evaluated by assessing nitric oxide (NO) production in LPS-activated BV-2 cells, a microglia cell line. Piperkadsin C (1) and futoquinol (2) potently inhibited NO production with an IC(50) value of 14.6 and 16.8microM in microglia cells, respectively. Compounds 3, 4, 5, 8, and 9 also exhibited moderate inhibition of NO production in BV-2 cells.
Pharmacokinetics of kadsurenone and its interaction with cyclosporin A in rats using a combined HPLC and microdialysis system.[Pubmed: 19111511]
Kadsurenone is a neolignan with specific antagonistic activity of platelet-activating factor, and is derived from the stems of Piper kadsura. To investigate the mechanism of hepatobiliary excretion of kadsurenone and its association with P-glycoprotein (P-gp), and to explore whether the hepatobiliary excretion of kadsurenone was associated with P-gp, a microdialysis system coupled with HPLC was developed to measure free-form kadsurenone in rat blood and bile. This study design was parallel in the following groups: six rats received kadsurenone alone (20 and 30 mg/kg, i.v.) as control group and the treated-group rats were co-administered with kadsurenone and CsA; P-gp inhibitor. The microdialysis probes were respectively inserted into the jugular vein toward right atrium and bile duct of male Sprague-Dawley rats for blood and bile sampling. CsA (20mg/kg) was administered 10 min prior to kadsurenone administration through the femoral vein and the collected samples were analyzed by a HPLC system. The analytes were separated by a C18 column (150 x 4.6 mm I.D., 5 microm) with a mobile phase of acetonitrile-water (50:50, v/v) at a flow-rate of 1 mL/min. The UV detection wavelength was set 235 nm. The calibration curve was linear over the concentration range of 0.05-10 microg/mL with the coefficient of determination of 0.997. The inter- and intra-assay accuracy and precision of the method ranged from -9.53% to 6.75%. The limit of detection and the limit of quantification were 0.01 and 0.05 microg/mL, respectively. The hepatobiliary excretion ratio of kadsurenone was defined by dividing the values of the area under the drug concentration curve (AUC) for bile and blood (AUC(bile)/AUC(blood)). The results indicated that the hepatobiliary excretion ratio of kadsurenone on the CsA treated-group was 1.2+/-0.1, which was not significantly different from the group of kadsurenone alone (1.3+/-0.2). This fact indicates that kadsurenone went through hepatobiliary excretion but might not be regulated by P-gp.


