(+)-Licarin ACAS# 51020-86-1 |
- (-)-licarin A
Catalog No.:BCN5087
CAS No.:23518-30-1
- Dehydrodiisoeugenol
Catalog No.:BCN1240
CAS No.:2680-81-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51020-86-1 | SDF | Download SDF |
| PubChem ID | 6442393 | Appearance | Powder |
| Formula | C20H22O4 | M.Wt | 326.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
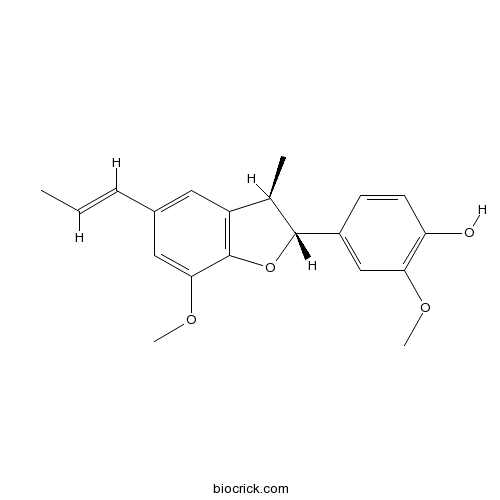
| Chemical Name | 2-methoxy-4-[(2R,3R)-7-methoxy-3-methyl-5-[(E)-prop-1-enyl]-2,3-dihydro-1-benzofuran-2-yl]phenol | ||
| SMILES | CC=CC1=CC2=C(C(=C1)OC)OC(C2C)C3=CC(=C(C=C3)O)OC | ||
| Standard InChIKey | ITDOFWOJEDZPCF-OTBILJLCSA-N | ||
| Standard InChI | InChI=1S/C20H22O4/c1-5-6-13-9-15-12(2)19(24-20(15)18(10-13)23-4)14-7-8-16(21)17(11-14)22-3/h5-12,19,21H,1-4H3/b6-5+/t12-,19-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(+)-Licarin A Dilution Calculator

(+)-Licarin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0637 mL | 15.3186 mL | 30.6373 mL | 61.2745 mL | 76.5931 mL |
| 5 mM | 0.6127 mL | 3.0637 mL | 6.1275 mL | 12.2549 mL | 15.3186 mL |
| 10 mM | 0.3064 mL | 1.5319 mL | 3.0637 mL | 6.1275 mL | 7.6593 mL |
| 50 mM | 0.0613 mL | 0.3064 mL | 0.6127 mL | 1.2255 mL | 1.5319 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6127 mL | 0.7659 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Demethylcephalotaxinone
Catalog No.:BCN7070
CAS No.:51020-45-2
- Isocorynoxeine
Catalog No.:BCN5003
CAS No.:51014-29-0
- 6''-O-Malonylgenistin
Catalog No.:BCN2772
CAS No.:51011-05-3
- Minecoside
Catalog No.:BCN5627
CAS No.:51005-44-8
- Galanthaminone
Catalog No.:BCN2867
CAS No.:510-77-0
- AMI-193
Catalog No.:BCC6679
CAS No.:510-74-7
- Echinocystic acid
Catalog No.:BCN5628
CAS No.:510-30-5
- Belladonnine
Catalog No.:BCN1892
CAS No.:510-25-8
- Voacangine
Catalog No.:BCN3224
CAS No.:510-22-5
- Trachelanthine
Catalog No.:BCN2042
CAS No.:510-19-0
- Tetramethylammonium
Catalog No.:BCN1816
CAS No.:51-92-3
- (2-Acetoxyethyl)trimethylammonium
Catalog No.:BCN1743
CAS No.:51-84-3
- (-)-Licarin B
Catalog No.:BCN1241
CAS No.:51020-87-2
- Salbutamol Sulfate
Catalog No.:BCC4338
CAS No.:51022-70-9
- Acipimox
Catalog No.:BCC4884
CAS No.:51037-30-0
- Flurbiprofen
Catalog No.:BCC3781
CAS No.:5104-49-4
- Z-GABA-OH,Z-gama-Abu-OH
Catalog No.:BCC2644
CAS No.:5105-78-2
- alpha-Lipomycin
Catalog No.:BCN1842
CAS No.:51053-40-8
- Wogonoside
Catalog No.:BCN1200
CAS No.:51059-44-0
- ATC 0065
Catalog No.:BCC7666
CAS No.:510732-84-0
- ATC 0175 hydrochloride
Catalog No.:BCC7657
CAS No.:510733-97-8
- Boc-Lys(Z)-pNA
Catalog No.:BCC3419
CAS No.:51078-31-0
- Pyrrolidinedithiocarbamate ammonium
Catalog No.:BCC6766
CAS No.:5108-96-3
- Methyl p-hydroxyphenyllactate
Catalog No.:BCN6669
CAS No.:51095-47-7
Matrix Solid-Phase Dispersion Combined with HPLC-DAD for Simultaneous Determination of Nine Lignans in Saururus chinensis.[Pubmed:30272133]
J Chromatogr Sci. 2019 Feb 1;57(2):186-193.
A simple and rapid method, based on matrix solid-phase dispersion (MSPD) and high-performance liquid chromatography (HPLC) was developed for simultaneous determination of nine lignans, including (-)-(7R,8R)-machilin D (Wang, C., Wang, P., Chen, X., Wang, W., Jin, Y.; Saururus chinensis (Lour.) Baill blocks enterovirus 71 infection by hijacking MEK1-ERK signaling pathway; Antiviral Research, (2015); 119:47-56), dihydroguaiaretic acid (Quan, Z., Lee, Y.J., Yang, J.H., Lu,Y., Li,Y., Lee,Y.K., et al.; Ethanol extracts of Saururus chinensis suppress ovalbumin-sensitization airway inflammation; Journal of Ethnopharmacology, (2010); 132:143-149.), sauchinone (Zhuang, T., Liang, J.Y., Sun, J.B., Wu, Y., Huang, L.R., Qu, W.; Secondary metabolites from Saururus chinensis and their chemotaxonomic significance; Biochemical Systematics and Ecology, (2014); 56:95-98.), rel-(7S,8S,7'R,8'R)-3,3',4,4',5,5'-hexamethoxy-7.O.7',8.8'-lignan (Tsai, W.J., Shen, C.C., Tsai, T.H., Lin, L.C.; Lignans from the aerial parts of Saururus chinensis: isolation, structural characterization, and their effects on platelet aggregation; Journal of Natural Products, (2014); 77:125-131), (+)-Licarin A (Cui, H., Xu, B., Wu, T., Xu, J., Yuan, Y., Gu, Q.; Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein-Barr virus lytic replication; Journal of Natural Products, (2014); 77:100-110.), manassantin A (Lu, Y., Piao, D., Zhang, H., Li, X., Chao, G.H., Park, S.J., et al.; Saucerneol F inhibits tumor necrosis factor-alpha and IL-6 production by suppressing Fyn-mediated pathways in FcepsilonRI-mediated mast cells; Food and Chemical Toxicology, (2013); 59:696-702.), saurucinol I (Kwon, O.E., Lee, H.S., Lee, S.W., Chung, M.Y., Bae, K.H., Rho, M.C., et al.; Manassantin A and B isolated from Saururus chinensis inhibit TNF-alpha-induced cell adhesion molecule expression of human umbilical vein endothelial cells; Archives of Pharmacal Research, (2005); 28:55-60.), manassantin B (Hwang, B.Y., Lee, J.H., Jung, H.S., Kim, K.S., Nam, J.B., Hong, Y.S., et al.; Sauchinone, a lignan from Saururus chinensis, suppresses iNOS expression through the inhibition of transactivation activity of RelA of NF-kappaB; Planta Medica, (2003); 69:1096-01.) and licarin B (Hwang, B.Y., Lee, J.H., Nam, J.B., Hong, Y.S., Lee, J.J.; Lignans from Saururus chinensis inhibiting the transcription factor NF-kappaB; Phytochemistry, (2003); 64:765-771.) in Saururus chinensis. The parameters of MSPD were optimized to be that 0.2 g of sample, blended with 0.4 g silica gel, and eluted with 5 mL of methanol. The separation was carried out on a C18 column with acidified aqueous acetonitrile gradients. The established method was fully validated in terms of linearity (r2 >/= 0.9994), sensitivity, precision (RSD
Licarin A induces cell death by activation of autophagy and apoptosis in non-small cell lung cancer cells.[Pubmed:29468481]
Apoptosis. 2018 Apr;23(3-4):210-225.
Lung cancer has a relatively poor prognosis with a low survival rate and drugs that target other cell death mechanism like autophagy may help improving current therapeutic strategy. This study investigated the anti-proliferative effect of (+)-Licarin A (LCA) from Myristica fragrans in non-small cell lung cancer cell lines-A549, NCI-H23, NCI-H520 and NCI-H460. LCA inhibited proliferation of all the four cell lines in a dose and time dependent manner with minimum IC50 of 20.03 +/- 3.12, 22.19 +/- 1.37 microM in NCI-H23 and A549 cells respectively. Hence NCI-H23 and A549 cells were used to assess the ability LCA to induce autophagy and apoptosis. LCA treatment caused G1 arrest, increase in Beclin 1, LC3II levels and degradation of p62 indicating activation of autophagy in both NCI-H23 and A549 cells. In addition, LCA mediated apoptotic cell death was confirmed by MMP loss, increased ROS, cleaved PARP and decreased pro-caspase3. To understand the role of LCA induced autophagy and its association with apoptosis, cells were analysed following treatment with a late autophagy inhibitor-chloroquine and also after Beclin 1 siRNA transfection. Data indicated that inhibition of autophagy resulted in reduced anti-proliferative as well as pro-apoptotic ability of LCA. These findings confirmed that LCA brought about autophagy dependent apoptosis in non-small cell lung cancer cells and hence it may serve as a potential drug candidate for non-small cell lung cancer therapy.
Distinctive effects of licarin A on lipolysis mediated by PKA and on formation of brown adipocytes from C3H10T1/2 mesenchymal stem cells.[Pubmed:29288687]
Toxicol Appl Pharmacol. 2018 Feb 1;340:9-20.
Obesity increases with the positive energy imbalance and correlates with increased risks for metabolic diseases. Promotion of white adipose tissue beiging has received considerable attention due to possible usefulness for preventing obesity and the comorbidities. (+)-Licarin A (LA) is a compound derived from Mexican medicinal plant Aristolochia taliscana. Here, we report that LA stimulates the development of brown-like and beige-like adipocytes from C3H10T1/2 mesenchymal stem cells with phenotypic shifts to formation of smaller lipid droplets. LA also markedly induced the expression of proteins characteristic of brown-like adipocytes in C3H10T1/2 mesenchymal stem cells. LA induced uncoupling protein 1 (Ucp1) and expression of other thermogenic genes in C3H10T1/2 mesenchymal stem cells via a mechanism involving protein kinase A (PKA). LA treatment also inhibited expression of white-adipocyte-specific genes. Moreover, LA treatment promoted lipolysis via PKA mediated pathway. Our findings inaugurate a new role of LA as an inducer of brown-like adipocytes formation with lipolytic properties, which in future might be studied in vivo as a potential anti-obesity agent.
Natural Compounds from Mexican Medicinal Plants as Potential Drug Leads for Anti-Tuberculosis Drugs.[Pubmed:28198919]
An Acad Bras Cienc. 2017 Jan-Mar;89(1):31-43.
In Mexican Traditional Medicine 187 plant species are used in the treatment of respiratory conditions that may be associated with tuberculosis. In this contribution, we review the ethnobotany, chemistry and pharmacology of 63 species whose extracts have been assayed for antimycobacterial activity in vitro. Among these, the most potent is Aristolochia brevipes (MIC= 12.5 microg/mL), followed by Aristolochia taliscana, Citrus sinensis, Chrysactinia mexicana, Persea americana, and Olea europaea (MIC<64 microg/mL). Other potent extracts (inhibition > 95%, 50 microg/mL) include: Amphipterygium adstringens, Larrea divaricata, and Phoradendron robinsoni. Several active compounds have been identified, the most potent are: (+)-Licarin A (isolated from A. taliscana), and 9-amino-9-methoxy-3,4-dihydro-2H-benzo[h]-chromen-2-one (transformation product of 9-methoxytariacuripyrone isolated from Aristolochia brevipes), both with MIC= 3.125 microg/mL, that is 8-fold less potent than the reference drug Rifampicin (MIC= 0.5 microg/mL). Any of the compounds or extracts here reviewed has been studied in clinical trials or with animal models; however, these should be accomplished since several are active against strains resistant to common drugs.
HPLC-Guided Isolation, Purification and Characterization of Phenylpropanoid and Phenolic Constituents of Nutmeg Kernel (Myristica fragrans).[Pubmed:27396199]
Nat Prod Commun. 2016 Apr;11(4):483-8.
Many studies on the biological activities of nutmeg continue to appear in the literature. The most common targets include GIT, CNS, oxidative stress and inflammation. However, results obtained from most studies are often inconsistent due to the variability of utilized samples, lack of standardized nutmeg products or insufficient amounts of pure compounds for comprehensive follow-up investigation. To address the consistency and supply issue we utilized available technology to develop a reproducible procedure for preparation of specific extracts and isolation of the major phenolic constituents present in nutmeg kemel. A well-defined and reproducible sequence of extraction, fractionation and chromatographic purification was adopted and was guided by HPLC fingerprinting. Spectroscopic methods, mainly NMR, and mass spectrometry were utilized to identify each compound. Thirteen compounds were isolated in a pure form and identified as: elemicin (1), isoelemicin (2), myristicin (4), surinamensin (5), malabaricone C (6), 2-(3'-allyl-2',6'-dimethoxy-phenyloxy)-l- acetoxy-(3,4-dimethoxyphenyl)-propyl ester (7), methoxylicarin A (8), (+)-Licarin A (9), malabaricone B (10), licarin C (11), 5'-methoxylicarin B (12), licarin B (13), and 2-(3'-allyl-2',6'-dimethoxy-phenyloxy)-l-methyl-5-methoxy-1,2-dihydrobenzofuran (3, a new compound). With repeated isolation runs, these pure compounds can be prepared in quantities sufficient for biological evaluation as needed. The availability of purified compounds will also allow the development of specific, accurate, and sensitive analytical procedures for pharmacokinetic studies and for quality control of nutmeg products. Both aspects are essential for nutmeg-focused drug discovery. The same approach can also be adapted to other medicinal plants of potential interest.
Licarin A is a candidate compound for the treatment of immediate hypersensitivity via inhibition of rat mast cell line RBL-2H3 cells.[Pubmed:26376734]
J Pharm Pharmacol. 2015 Dec;67(12):1723-32.
OBJECTIVES: We previously demonstrated that some phenylpropanoids are capable of inhibiting activated mast cells. This study evaluated the anti-allergic effects of (+)-Licarin A, a neolignan isolated from various plants, on antigen-stimulated rat mast cell line. METHODS: The inhibitory effects of (+)-Licarin A on histamine release, tumour necrosis factor-alpha (TNF-alpha) and prostaglandin D2 (PGD2) production, and cyclooxygenase-2 (COX-2) expression in dinitrophenyl-human serum albumin (DNP-HSA) rat basophilic leukemia cells (DNP-HSA-stimulated RBL-2H3 cells), were investigated by spectrofluorometry, ELISA and immunoblotting. KEY FINDINGS: (+)-Licarin A significantly and dose-dependently reduced TNF-alpha production (IC50 12.6 +/- 0.3 mum) in DNP-HSA-stimulated RBL-2H3 cells. Furthermore, the levels of PGD2 secretion in DNP-HSA-stimulated cells pretreated with (+)-Licarin A were lower than those stimulated with DNP-HSA alone (positive control). Treatment with (+)-Licarin A at 20 mum produced slight suppression of DNP-HSA-induced increases in COX-2 mRNA and protein levels. We identified several signalling pathways that mediated these pharmacological effects. (+)-Licarin A treatment tended to reduce phosphorylated protein kinase C alpha/beta II (PKCalpha/betaII) and p38 mitogen-activated protein kinase (MAPK) protein levels. CONCLUSIONS: Our results demonstrate that (+)-Licarin A reduces TNF-alpha and PGD2 secretion via the inhibition of PKCalpha/betaII and p38 MAPK pathways; this compound may be useful for attenuating immediate hypersensitivity.
Biological evaluation of secondary metabolites from the root of Machilus obovatifolia.[Pubmed:26172326]
Chem Biodivers. 2015 Jul;12(7):1057-67.
Bioassay-guided fractionation of the root of Machilus obovatifolia led to the isolation of four new lignans, epihenricine B (1), threo-(7'R,8'R) and threo-(7'S,8'S)-methylmachilusol D (2 and 3), and isofragransol A (4), along with 23 known compounds. The compounds were obtained as isomeric mixtures (i.e., 2/3 and 4/20, resp.). The structures were elucidated by spectral analyses. Among the isolates, 1, (+)-Licarin A (12), guaiacin (14), (+/-)-syringaresinol (21), and (-)-epicatechin (23) showed ABTS (=2,2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) cation radical-scavenging activity, with SC50 values of 11.7+/-0.5, 12.3+/-1.1, 11.0+/-0.1, 10.6+/-0.3, and 9.5+/-0.2 muM in 20 min, respectively. In addition, kachirachirol B (17) showed cytotoxicity against the NCI-H460 cell line with an IC50 value of 3.1 mug/ml.
Neolignans from Aristolochia elegans as antagonists of the neurotropic effect of scorpion venom.[Pubmed:25278184]
J Ethnopharmacol. 2014 Nov 18;157:156-60.
ETHNOPHARMACOLOGICAL RELEVANCE: The high frequency of poisoning by sting or bite from venomous animals has begun to be a serious public health problem in Mexico where scorpion sting is the most common. Because of this, there is the need to seek active substances in plant species with an antagonistic effect against neurotropic activity of scorpion venom. The aim of this work was to demonstrate which of the compounds contained in the n-hexane extract from Aristolochia elegans roots display activity against scorpion venom. MATERIAL AND METHODS: Antagonist activity displayed by extract, fractions and isolated compounds obtained from Aristolochia elegans was guided by the inhibition of smooth muscle contraction induced by scorpion venom (Centruroides limpidus limpidus) in a model of isolated guinea pig ileum. The neolignans obtained from this extract were isolated and analyzed by chromatographic methods including HPLC. The chemical characterization of these compounds was performed by the analysis of (1)H and (13)C NMR spectra. RESULTS: The bio-guided chromatographic fractionation allowed us to isolate 4 known neolignans: Eupomatenoid-7 (1), (+)-Licarin A (2), licarin B (3), eupomatenoid-1 (4) and other new neolignan which was characterized as 2-(3'-hydroxy-4'-methoxyphenyl)-3-methyl-5-[(E)-alpha-propen-gamma-al]-7-methoxy- benzo [b] furan (5). This compound was named as eleganal. Compounds 1 and 2 were purified from the most active fraction AeF3 (EC50 of 149.9mug/mL, Emax of 65.66%). A doses-response analysis of eupomatenoid-7(1) and (+)-Licarin A(2) allowed us to establish EC50 values (65.96mug/mL and 51.96mug/mL) respectively. CONCLUSIONS: The antagonistic effect against Centuroides limpidus limpidus scorpion venom displayed by the n-hexane extract from Aristolochia elegans roots is due to the presence of neolignans 1-2 contained in the fraction AeF3. Chemical analysis of fraction AeF2 allowed the isolation of a new compound which was identified as 2-(3'-hydroxy-4'-methoxyphenyl)-3-methyl-5-[(E)-alpha-propen-gamma-al]-7-methoxy- benzo[b]furan (5), denominated as eleganal.
Neolignan Licarin A presents effect against Leishmania (Leishmania) major associated with immunomodulation in vitro.[Pubmed:23891943]
Exp Parasitol. 2013 Oct;135(2):307-13.
Leishmaniasis' treatment is based mostly on pentavalent antimonials or amphotericin B long-term administration, expensive drugs associated with severe side effects. Considering these aforementioned, the search for alternative effective and safe leishmaniasis treatments is a necessity. This work evaluated a neolignan, (+)-Licarin A anti-leishmanial activity chemically synthesized by our study group. It was observed that (+)-Licarin A effectively inhibited Leishmania (Leishmania) major promastigotes (IC(5)(0) of 9.59 +/- 0.94 mug/mL) growth, by inducing in these parasites genomic DNA fragmentation in a typical death pattern by apoptosis. Additionally, the neolignan proved to be even more active against intracellular amastigotes of the parasite (EC(5)(0) of 4.71 +/- 0.29 mug/mL), and significantly more effective than meglumine antimoniate (EC(5)(0) of 216.2 +/- 76.7 mug/mL) used as reference drug. The antiamastigote activity is associated with an immunomodulatory activity, since treatment with (+)-Licarin A of the infected macrophages induced a decrease in the interleukin (IL)-6 and IL-10 production. This study demonstrates for the first time the antileishmanial activity of (+)-Licarin A and suggests that the compound may be a promising in the development of a new leishmanicidal agent.


