alpha-LipomycinCAS# 51053-40-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51053-40-8 | SDF | Download SDF |
| PubChem ID | 76185229 | Appearance | Red powder |
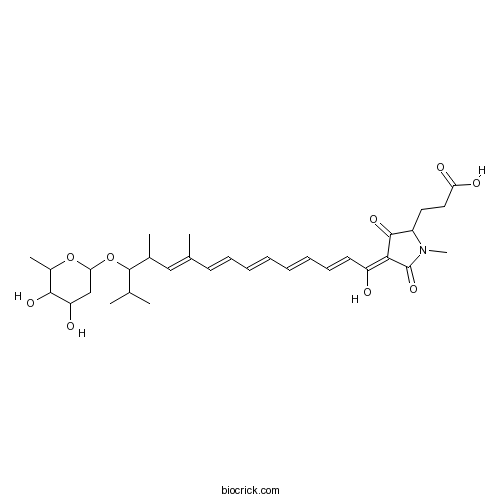
| Formula | C32H45NO9 | M.Wt | 587.71 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-[4-[13-(4,5-dihydroxy-6-methyloxan-2-yl)oxy-1-hydroxy-10,12,14-trimethylpentadeca-2,4,6,8,10-pentaenylidene]-1-methyl-3,5-dioxopyrrolidin-2-yl]propanoic acid | ||
| SMILES | CC1C(C(CC(O1)OC(C(C)C)C(C)C=C(C)C=CC=CC=CC=CC(=C2C(=O)C(N(C2=O)C)CCC(=O)O)O)O)O | ||
| Standard InChIKey | BRPRNHPFSOESDI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C32H45NO9/c1-19(2)31(42-27-18-25(35)29(38)22(5)41-27)21(4)17-20(3)13-11-9-7-8-10-12-14-24(34)28-30(39)23(15-16-26(36)37)33(6)32(28)40/h7-14,17,19,21-23,25,27,29,31,34-35,38H,15-16,18H2,1-6H3,(H,36,37) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Alpha-Lipomycin belongs to the classification of hybrid peptide-polyketide natural products. 2. Alpha-Lipomycin is active against gram-positive organisms. |
| Targets | Antifection |

alpha-Lipomycin Dilution Calculator

alpha-Lipomycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7015 mL | 8.5076 mL | 17.0152 mL | 34.0304 mL | 42.538 mL |
| 5 mM | 0.3403 mL | 1.7015 mL | 3.403 mL | 6.8061 mL | 8.5076 mL |
| 10 mM | 0.1702 mL | 0.8508 mL | 1.7015 mL | 3.403 mL | 4.2538 mL |
| 50 mM | 0.034 mL | 0.1702 mL | 0.3403 mL | 0.6806 mL | 0.8508 mL |
| 100 mM | 0.017 mL | 0.0851 mL | 0.1702 mL | 0.3403 mL | 0.4254 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Z-GABA-OH,Z-gama-Abu-OH
Catalog No.:BCC2644
CAS No.:5105-78-2
- Flurbiprofen
Catalog No.:BCC3781
CAS No.:5104-49-4
- Acipimox
Catalog No.:BCC4884
CAS No.:51037-30-0
- Salbutamol Sulfate
Catalog No.:BCC4338
CAS No.:51022-70-9
- (-)-Licarin B
Catalog No.:BCN1241
CAS No.:51020-87-2
- (+)-Licarin A
Catalog No.:BCC9008
CAS No.:51020-86-1
- Demethylcephalotaxinone
Catalog No.:BCN7070
CAS No.:51020-45-2
- Isocorynoxeine
Catalog No.:BCN5003
CAS No.:51014-29-0
- 6''-O-Malonylgenistin
Catalog No.:BCN2772
CAS No.:51011-05-3
- Minecoside
Catalog No.:BCN5627
CAS No.:51005-44-8
- Galanthaminone
Catalog No.:BCN2867
CAS No.:510-77-0
- AMI-193
Catalog No.:BCC6679
CAS No.:510-74-7
- Wogonoside
Catalog No.:BCN1200
CAS No.:51059-44-0
- ATC 0065
Catalog No.:BCC7666
CAS No.:510732-84-0
- ATC 0175 hydrochloride
Catalog No.:BCC7657
CAS No.:510733-97-8
- Boc-Lys(Z)-pNA
Catalog No.:BCC3419
CAS No.:51078-31-0
- Pyrrolidinedithiocarbamate ammonium
Catalog No.:BCC6766
CAS No.:5108-96-3
- Methyl p-hydroxyphenyllactate
Catalog No.:BCN6669
CAS No.:51095-47-7
- 2,7-Dihydrohomoerysotrine
Catalog No.:BCN5629
CAS No.:51095-85-3
- Nicotine 1'-N-oxide
Catalog No.:BCN6892
CAS No.:51095-86-4
- alpha-Onocerol
Catalog No.:BCN5630
CAS No.:511-01-3
- Sugiol
Catalog No.:BCN3164
CAS No.:511-05-7
- Totarol
Catalog No.:BCN4627
CAS No.:511-15-9
- Vitamin D4
Catalog No.:BCC2042
CAS No.:511-28-4
alpha- and beta-Lipomycin: total syntheses by sequential stille couplings and assignment of the absolute configuration of all stereogenic centers.[Pubmed:24895187]
Angew Chem Int Ed Engl. 2014 Jul 7;53(28):7328-34.
40 years ago spectroscopy, derivatization, and degradation revealed the structures of alpha-Lipomycin and its aglycon beta-lipomycin except for the configurations of their side-chain stereocenters. We synthesized all relevant beta-lipomycin candidates: the (12R,13S) isomer has the same specific rotational value as the natural product. By the same criterion the (12R,13S)-configured D-digitoxide is identical to alpha-Lipomycin. We double-checked our assignments by degrading alpha- and beta-lipomycin to the diesters 33 and 34 and proving their 3D structures synthetically.
Discovery of a new family of Dieckmann cyclases essential to tetramic acid and pyridone-based natural products biosynthesis.[Pubmed:25621700]
Org Lett. 2015 Feb 6;17(3):628-31.
Bioinformatic analyses indicate that TrdC, SlgL, LipX2, KirHI, and FacHI belong to a group of highly homologous proteins involved in biosynthesis of actinomycete-derived tirandamycin B, streptolydigin, alpha-Lipomycin, kirromycin, and factumycin, respectively. However, assignment of their biosynthetic roles has remained elusive. Gene inactivation and complementation, in vitro biochemical assays with synthetic analogues, point mutations, and phylogenetic tree analyses reveal that these proteins represent a new family of Dieckmann cyclases that drive tetramic acid and pyridone scaffold biosynthesis.
Biosynthetic gene cluster for the polyenoyltetramic acid alpha-lipomycin.[Pubmed:16723573]
Antimicrob Agents Chemother. 2006 Jun;50(6):2113-21.
The gram-positive bacterium Streptomyces aureofaciens Tu117 produces the acyclic polyene antibiotic alpha-Lipomycin. The entire biosynthetic gene cluster (lip gene cluster) was cloned and characterized. DNA sequence analysis of a 74-kb region revealed the presence of 28 complete open reading frames (ORFs), 22 of them belonging to the biosynthetic gene cluster. Central to the cluster is a polyketide synthase locus that encodes an eight-module system comprised of four multifunctional proteins. In addition, one ORF shows homology to those for nonribosomal peptide synthetases, indicating that alpha-Lipomycin belongs to the classification of hybrid peptide-polyketide natural products. Furthermore, the lip cluster includes genes responsible for the formation and attachment of d-digitoxose as well as ORFs that resemble those for putative regulatory and export functions. We generated biosynthetic mutants by insertional gene inactivation. By analysis of culture extracts of these mutants, we could prove that, indeed, the genes involved in the biosynthesis of lipomycin had been cloned, and additionally we gained insight into an unusual biosynthesis pathway.


