AMI-1935-HT/D2DR receptor antagonist CAS# 510-74-7 |
- Nutlin-3a chiral
Catalog No.:BCC1812
CAS No.:675576-98-4
- p53 and MDM2 proteins-interaction-inhibitor chiral
Catalog No.:BCC1830
CAS No.:939981-37-0
- p53 and MDM2 proteins-interaction-inhibitor racemic
Catalog No.:BCC1831
CAS No.:939983-14-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 510-74-7 | SDF | Download SDF |
| PubChem ID | 77445 | Appearance | Powder |
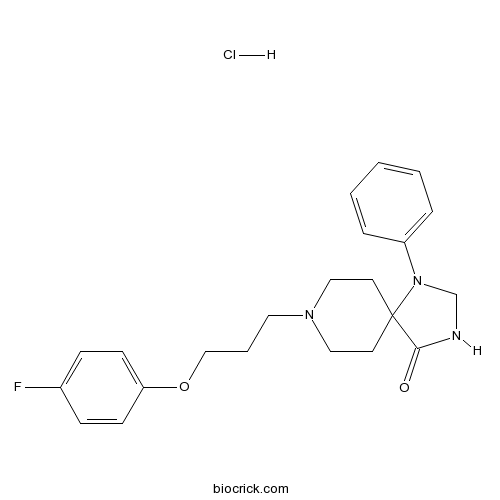
| Formula | C22H26FN3O2 | M.Wt | 383.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 75 mM in DMSO | ||
| Chemical Name | 8-[3-(4-fluorophenoxy)propyl]-1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one;hydrochloride | ||
| SMILES | C1CN(CCC12C(=O)NCN2C3=CC=CC=C3)CCCOC4=CC=C(C=C4)F.Cl | ||
| Standard InChIKey | WEEQZERYOOQWBZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H26FN3O2.ClH/c23-18-7-9-20(10-8-18)28-16-4-13-25-14-11-22(12-15-25)21(27)24-17-26(22)19-5-2-1-3-6-19;/h1-3,5-10H,4,11-17H2,(H,24,27);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective 5-HT antagonist, which binds to 5-HT2 sites as potently as spiperone but has lower affinity for 5-HT2C receptors. Also a high affinity D2 receptor antagonist (Ki = 3 nM). Lacks the disruptive effect of spiperone on animal behavior. |

AMI-193 Dilution Calculator

AMI-193 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6078 mL | 13.0392 mL | 26.0783 mL | 52.1567 mL | 65.1958 mL |
| 5 mM | 0.5216 mL | 2.6078 mL | 5.2157 mL | 10.4313 mL | 13.0392 mL |
| 10 mM | 0.2608 mL | 1.3039 mL | 2.6078 mL | 5.2157 mL | 6.5196 mL |
| 50 mM | 0.0522 mL | 0.2608 mL | 0.5216 mL | 1.0431 mL | 1.3039 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2608 mL | 0.5216 mL | 0.652 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AMI-193 is a potent and selective antagonist of 5-HT2A receptor and dopamine D2-receptor with Ki values of 2 and 3 nM, respectively [1].
The 5-HT2A receptor is a G protein-coupled receptor and a subtype of the 5-HT2 receptor. The 5-HT2A receptor plays an important role in the spread of the human polyoma virus. The dopamine D2-receptor is a G protein-coupled receptor that inhibits adenylyl cyclase activity.
AMI-193 is a potent and selective 5-HT2A receptor and dopamine D2-receptor antagonist. AMI-193 was highly selective for 5-HT2A versus 5-HT2C receptor with Ki values of 2 and 4300 nM for 5-HT2A and 5-HT2C receptor, respectively [1]. Also, AMI-193 bound to 5-HT2 receptor with Ki value of 2 nM and exhibited >2000-fold selectivity versus 5-HT1C receptor. In cortical slices, AMI-193 (10-10 - 10-5 M) inhibited GABA release in a dose-dependent way and increased 5-HT outflow [3].
In rats, AMI-193 behaved as an antagonist with ED50 value of 0.003 mg/kg [2].
References:
[1]. Czoty PW, Howell LL. Behavioral effects of AMI-193, a 5-HT(2A)- and dopamine D(2)-receptor antagonist, in the squirrel monkey. Pharmacol Biochem Behav, 2000, 67(2): 257-264.
[2]. Ismaiel AM, De Los Angeles J, Teitler M, et al. Antagonism of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane stimulus with a newly identified 5-HT2- versus 5-HT1C-selective antagonist. J Med Chem, 1993, 36(17): 2519-2525.
[3]. Luparini MR, Garrone B, Pazzagli M, et al. A cortical GABA-5HT interaction in the mechanism of action of the antidepressant trazodone. Prog Neuropsychopharmacol Biol Psychiatry, 2004, 28(7): 1117-1127.
- Echinocystic acid
Catalog No.:BCN5628
CAS No.:510-30-5
- Belladonnine
Catalog No.:BCN1892
CAS No.:510-25-8
- Voacangine
Catalog No.:BCN3224
CAS No.:510-22-5
- Trachelanthine
Catalog No.:BCN2042
CAS No.:510-19-0
- Tetramethylammonium
Catalog No.:BCN1816
CAS No.:51-92-3
- (2-Acetoxyethyl)trimethylammonium
Catalog No.:BCN1743
CAS No.:51-84-3
- Carbamoylcholine chloride
Catalog No.:BCC7492
CAS No.:51-83-2
- Tyramine
Catalog No.:BCN6776
CAS No.:51-67-2
- 4'-Methoxyacetanilide
Catalog No.:BCC8711
CAS No.:51-66-1
- D-Amphetamine sulfate
Catalog No.:BCC5942
CAS No.:51-63-8
- Homatropine Bromide
Catalog No.:BCC4570
CAS No.:51-56-9
- Atropine
Catalog No.:BCN5639
CAS No.:51-55-8
- Galanthaminone
Catalog No.:BCN2867
CAS No.:510-77-0
- Minecoside
Catalog No.:BCN5627
CAS No.:51005-44-8
- 6''-O-Malonylgenistin
Catalog No.:BCN2772
CAS No.:51011-05-3
- Isocorynoxeine
Catalog No.:BCN5003
CAS No.:51014-29-0
- Demethylcephalotaxinone
Catalog No.:BCN7070
CAS No.:51020-45-2
- (+)-Licarin A
Catalog No.:BCC9008
CAS No.:51020-86-1
- (-)-Licarin B
Catalog No.:BCN1241
CAS No.:51020-87-2
- Salbutamol Sulfate
Catalog No.:BCC4338
CAS No.:51022-70-9
- Acipimox
Catalog No.:BCC4884
CAS No.:51037-30-0
- Flurbiprofen
Catalog No.:BCC3781
CAS No.:5104-49-4
- Z-GABA-OH,Z-gama-Abu-OH
Catalog No.:BCC2644
CAS No.:5105-78-2
- alpha-Lipomycin
Catalog No.:BCN1842
CAS No.:51053-40-8
Behavioral effects of AMI-193, a 5-HT(2A)- and dopamine D(2)-receptor antagonist, in the squirrel monkey.[Pubmed:11124389]
Pharmacol Biochem Behav. 2000 Oct;67(2):257-64.
8-[3-(4-Fluorophenoxy) propyl]-1-phenyl-1,3,8-triazaspiro[4, 5]decan-4-one (AMI-193) was developed as a 5-HT(2A)-selective antagonist with in vivo activity suitable for behavioral studies. However, AMI-193 is a potent dopamine D(2)-receptor antagonist with low nanomolar affinity. Accordingly, D(2)-actions may contribute to its behavioral pharmacology. In the present study, the effects of AMI-193 on operant behavior were characterized in squirrel monkeys. In subjects trained under a fixed-interval (FI) schedule of stimulus termination, AMI-193 (0.003-0.01 mg/kg) dose-dependently decreased response rate. When administered in combination with cocaine (0.03-3. 0 mg/kg) or the selective dopamine uptake inhibitor, GBR 12909 (0. 03-3.0 mg/kg), the rate-decreasing effects of AMI-193 were reversed by both dopamine indirect agonists. In drug-discrimination experiments, AMI-193 (0.003 and 0.01 mg/kg) attenuated the discriminative-stimulus effects of cocaine. AMI-193 (0.003 and 0.01 mg/kg) also reduced response rate under a second-order schedule of i. v. self-administration of cocaine (0.1 mg/infusion). The profile of behavioral effects and drug interactions observed in the present study, in conjunction with the relatively high affinity of AMI-193 for dopamine D(2) receptors, suggests that its D(2)-antagonist effects play a prominent role in the behavioral pharmacology of AMI-193.
Antagonism of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane stimulus with a newly identified 5-HT2- versus 5-HT1C-selective antagonist.[Pubmed:8355253]
J Med Chem. 1993 Aug 20;36(17):2519-25.
DOM [i.e., 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane] is a 5-HT1C/2 serotonin agonist that exerts stimulus control of behavior in animals. In order to determine if the discriminative stimulus effect of DOM is 5-HT1C- or 5-HT2-mediated, it would be informative to conduct tests of stimulus antagonism with a 5-HT1C- or 5-HT2-selective antagonist. To date, no such agents exist. Although the neuroleptic agent spiperone binds at D2 dopamine receptors and 5-HT1A serotonin receptors, (a) it displays about a 1000-fold selectivity for 5-HT2 versus 5-HT1C sites and (b) it has been used as a "5-HT2-selective" antagonist. Because spiperone is a behaviorally disruptive agent, it is not suitable for use in drug-discrimination studies. Using the spiperone molecule as a starting point, a limited structure-affinity investigation was conducted in order to identify a suitable antagonist with high affinity and selectivity for 5-HT2 receptors, and yet an antagonist that might lack the disruptive actions of spiperone. Various modifications of the spiperone molecule were examined, but most resulted in decreased 5-HT2 affinity or in loss of selectivity. One compound, 8-[3-(4-fluorophenoxy)propyl]-1-phenyl-1,3,8-triazaspiro[4.5]de can-4-on e (26), was shown to bind at 5-HT2 sites with high affinity (Ki = 2 nM) and > 2,000-fold selectivity versus 5-HT1C sites. In tests of stimulus antagonism using rats trained to discriminate 1 mg/kg of DOM from saline vehicle, 26 behaved as a potent antagonist (ED50 = 0.003 mg/kg) and lacked the disruptive effects associated with spiperone. As such, (a) it would appear that the DOM stimulus is primarily a 5-HT2-mediated, and not 5-HT1C-mediated, phenomenon, and (b) compound 26 may find application in other pharmacologic investigations where spiperone may not be a suitable antagonist.


