(-)-licarin ACAS# 23518-30-1 |
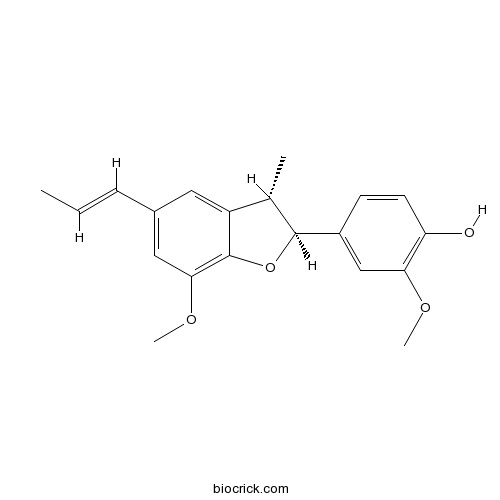
- Dehydrodiisoeugenol
Catalog No.:BCN1240
CAS No.:2680-81-1
- (+)-Licarin A
Catalog No.:BCC9008
CAS No.:51020-86-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23518-30-1 | SDF | Download SDF |
| PubChem ID | 5281836 | Appearance | Powder |
| Formula | C20H22O4 | M.Wt | 326.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-methoxy-4-[(2S,3S)-7-methoxy-3-methyl-5-[(E)-prop-1-enyl]-2,3-dihydro-1-benzofuran-2-yl]phenol | ||
| SMILES | CC=CC1=CC2=C(C(=C1)OC)OC(C2C)C3=CC(=C(C=C3)O)OC | ||
| Standard InChIKey | ITDOFWOJEDZPCF-FNINDUDTSA-N | ||
| Standard InChI | InChI=1S/C20H22O4/c1-5-6-13-9-15-12(2)19(24-20(15)18(10-13)23-4)14-7-8-16(21)17(11-14)22-3/h5-12,19,21H,1-4H3/b6-5+/t12-,19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Licarin A and (-)-Licarin A are promising compounds that could be used for the development of schistosomicidal and trypanocidal agents; Licarin A presents effect against Leishmania (Leishmania) major associated with immunomodulation in vitro; (-)-Licarin A has antimycobacterial activity, represents a potentially active anti-tuberculosis agent to treat MDR M. tuberculosis and NTM strains.Licarin A significantly protects primary cultured neuronal cells against glutamate-induced oxidative stress, via antioxidative activities. |
| Targets | DNA/RNA Synthesis | IL Receptor | Antifection |
| In vitro | Meso-dihydroguaiaretic acid and licarin A of Machilus thunbergii protect against glutamate-induced toxicity in primary cultures of a rat cortical cells.[Pubmed: 16151440]Br J Pharmacol. 2005 Nov;146(5):752-9.We previously reported that four lignans isolated from the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae) protected primary cultures of rat cortical neurons from neurotoxicity induced by glutamate. Neolignan Licarin A presents effect against Leishmania (Leishmania) major associated with immunomodulation in vitro.[Pubmed: 23891943]Exp Parasitol. 2013 Oct;135(2):307-13.Leishmaniasis' treatment is based mostly on pentavalent antimonials or amphotericin B long-term administration, expensive drugs associated with severe side effects. Considering these aforementioned, the search for alternative effective and safe leishmaniasis treatments is a necessity. Schistosomicidal and trypanocidal structure-activity relationships for (±)-licarin A and its (-)- and (+)-enantiomers.[Pubmed: 21570099]Phytochemistry. 2011 Aug;72(11-12):1424-30.(±)-Licarin A (1) was obtained by oxidative coupling, and its enantiomers, (-)-Licarin A (2) and (+)-Licarin A (3), were resolved by chiral HPLC.
|
| Animal Research | Antitubercular activity and the subacute toxicity of (-)-Licarin A in BALB/c mice: a neolignan isolated from Aristolochia taliscana.[Pubmed: 23291382]Arch Med Res. 2013 Feb;44(2):99-104.(-)-Licarin A (LA) was isolated from diverse plants such as Aristolochia taliscana and possesses antimycobacterial, antiinflammatory, trypanocidal, and neuroprotective activities. The aim of the study was to determine the antitubercular and subacute toxicity of (-)-Licarin A isolated from A. taliscana in BALB/c mice. |

(-)-licarin A Dilution Calculator

(-)-licarin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0637 mL | 15.3186 mL | 30.6373 mL | 61.2745 mL | 76.5931 mL |
| 5 mM | 0.6127 mL | 3.0637 mL | 6.1275 mL | 12.2549 mL | 15.3186 mL |
| 10 mM | 0.3064 mL | 1.5319 mL | 3.0637 mL | 6.1275 mL | 7.6593 mL |
| 50 mM | 0.0613 mL | 0.3064 mL | 0.6127 mL | 1.2255 mL | 1.5319 mL |
| 100 mM | 0.0306 mL | 0.1532 mL | 0.3064 mL | 0.6127 mL | 0.7659 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 10-Gingerol
Catalog No.:BCN5922
CAS No.:23513-15-7
- 6-Gingerol
Catalog No.:BCN1030
CAS No.:23513-14-6
- 8-Gingerol
Catalog No.:BCN5921
CAS No.:23513-08-8
- Humulon
Catalog No.:BCC8186
CAS No.:23510-81-8
- MEN 11270
Catalog No.:BCC6094
CAS No.:235082-52-7
- Axillaridine
Catalog No.:BCN2060
CAS No.:23506-96-9
- Peimine
Catalog No.:BCN1094
CAS No.:23496-41-5
- Hoechst 33258 analog 5
Catalog No.:BCC1627
CAS No.:23491-55-6
- Hoechst 33258 analog 2
Catalog No.:BCC1625
CAS No.:23491-54-5
- Hoechst 33342
Catalog No.:BCC1629
CAS No.:23491-52-3
- Hoechst 33258
Catalog No.:BCC1623
CAS No.:23491-45-4
- 2-Amino-5-mercapto-1,3,4-thiadiazole
Catalog No.:BCC8536
CAS No.:2349-67-9
- Vomifoliol
Catalog No.:BCN5088
CAS No.:23526-45-6
- 5-Aza-2'-deoxycytidine
Catalog No.:BCN2169
CAS No.:2353-33-5
- 8-Debenzoylpaeoniflorin
Catalog No.:BCC8787
CAS No.:23532-11-8
- Daunorubicin HCl
Catalog No.:BCC5083
CAS No.:23541-50-6
- Hoechst 33258 analog 3
Catalog No.:BCC1626
CAS No.:23554-98-5
- HOE 32020
Catalog No.:BCC1620
CAS No.:23554-99-6
- Hoechst 34580
Catalog No.:BCC1632
CAS No.:23555-00-2
- Procyanidin B3
Catalog No.:BCN6316
CAS No.:23567-23-9
- Clotrimazole
Catalog No.:BCC3755
CAS No.:23593-75-1
- Norisoboldine
Catalog No.:BCN6285
CAS No.:23599-69-1
- H-Phe-pNA
Catalog No.:BCC3010
CAS No.:2360-97-6
- [Arg14,Lys15]Nociceptin
Catalog No.:BCC5781
CAS No.:236098-40-1
Schistosomicidal and trypanocidal structure-activity relationships for (+/-)-licarin A and its (-)- and (+)-enantiomers.[Pubmed:21570099]
Phytochemistry. 2011 Aug;72(11-12):1424-30.
(+/-)-Licarin A (1) was obtained by oxidative coupling, and its enantiomers, (-)-licarin A (2) and (+)-licarin A (3), were resolved by chiral HPLC. Schistosomicidal and trypanocidal activities of these compounds were evaluated in vitro against Schistosoma mansoni adult worms and trypomastigote forms of Trypanosoma cruzi. The racemic mixture (1) displayed significant schistosomicidal activity with an LC(5)(0) value of 53.57 muM and moderate trypanocidal activity with an IC(5)(0) value of 127.17 muM. On the other hand, the (-)-enantiomer (2), displaying a LC(5)(0) value of 91.71 muM, was more active against S. mansoni than the (+)-enantiomer (3), which did not show activity. For the trypanocidal assay, enantiomer 2 showed more significant activity (IC(5)(0) of 23.46 muM) than enantiomer 3, which showed an IC(5)(0) value of 87.73 muM. Therefore, these results suggest that (+/-)-licarin A (1) and (-)-licarin A (2) are promising compounds that could be used for the development of schistosomicidal and trypanocidal agents.
Antitubercular activity and the subacute toxicity of (-)-Licarin A in BALB/c mice: a neolignan isolated from Aristolochia taliscana.[Pubmed:23291382]
Arch Med Res. 2013 Feb;44(2):99-104.
BACKGROUND AND AIMS: Tuberculosis remains a worldwide health problem and requires long-term treatment with several antibiotics; therefore, compliance problems and the emergence of multidrug resistance (MDR) are involved. (-)-licarin A (LA) was isolated from diverse plants such as Aristolochia taliscana and possesses antimycobacterial, antiinflammatory, trypanocidal, and neuroprotective activities. The aim of the study was to determine the antitubercular and subacute toxicity of LA isolated from A. taliscana in BALB/c mice. METHODS: The antitubercular activity of LA was tested in a TB murine model inducing disease with M. tuberculosis H37Rv or MDR. Mice were treated with LA (5 mg/kg) for 30 and 60 days; post/treatment, lung bacilli loads and pneumonia percentage were determined. The subacute toxicity of LA (21 days) was evaluated in healthy mice. After treatment, biochemical and hematological parameters were determined and main organs were analyzed histologically. RESULTS: In animals infected with drug-sensitive or MDR strains, LA produced a significant decrease of pulmonary bacillary burdens at day 30 of treatment, and a significant pneumonia reduction at days 30 and 60 of treatment. Regarding subacute toxicity, LA administration during 21 days showed no abnormalities in main-organ macro- and microarchitecture. Biochemical and hematological parameters analyzed showed no statistical differences between control and treated groups. CONCLUSIONS: (-)-licarin A reduces pneumonia of mice infected with both mycobacterium strains. Also, subacute toxicity of LA exhibits no major signs of damage. Biochemical and hematological parameters and histological analyses indicate that LA caused no significant changes at the doses assayed.
Meso-dihydroguaiaretic acid and licarin A of Machilus thunbergii protect against glutamate-induced toxicity in primary cultures of a rat cortical cells.[Pubmed:16151440]
Br J Pharmacol. 2005 Nov;146(5):752-9.
1 We previously reported that four lignans isolated from the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae) protected primary cultures of rat cortical neurons from neurotoxicity induced by glutamate. 2 Among the lignans, meso-dihydroguaiarectic acid (MDGA) and licarin A significantly attenuated glutamate-induced neurotoxicity when added prior to or right after the excitotoxic glutamate challenge. 3 The neuroprotective activities of two lignans appeared to be more effective in protecting neurons against neurotoxicity induced by NMDA than that induced by kainic acid. 4 MDGA and licarin A diminished the calcium influx that routinely accompanies with the glutamate-induced neurotoxicity, and inhibited the subsequent overproduction of cellular nitric oxide and peroxide to the level of control cells. They also preserved cellular activities of antioxidative enzymes such as superoxide dismutase, glutathione peroxidase and glutathione reductase reduced in the glutamate-injured neuronal cells. 5 Thus, our results suggest that MDGA and licarin A significantly protect primary cultured neuronal cells against glutamate-induced oxidative stress, via antioxidative activities.
Neolignan Licarin A presents effect against Leishmania (Leishmania) major associated with immunomodulation in vitro.[Pubmed:23891943]
Exp Parasitol. 2013 Oct;135(2):307-13.
Leishmaniasis' treatment is based mostly on pentavalent antimonials or amphotericin B long-term administration, expensive drugs associated with severe side effects. Considering these aforementioned, the search for alternative effective and safe leishmaniasis treatments is a necessity. This work evaluated a neolignan, licarin A anti-leishmanial activity chemically synthesized by our study group. It was observed that licarin A effectively inhibited Leishmania (Leishmania) major promastigotes (IC(5)(0) of 9.59 +/- 0.94 mug/mL) growth, by inducing in these parasites genomic DNA fragmentation in a typical death pattern by apoptosis. Additionally, the neolignan proved to be even more active against intracellular amastigotes of the parasite (EC(5)(0) of 4.71 +/- 0.29 mug/mL), and significantly more effective than meglumine antimoniate (EC(5)(0) of 216.2 +/- 76.7 mug/mL) used as reference drug. The antiamastigote activity is associated with an immunomodulatory activity, since treatment with licarin A of the infected macrophages induced a decrease in the interleukin (IL)-6 and IL-10 production. This study demonstrates for the first time the antileishmanial activity of licarin A and suggests that the compound may be a promising in the development of a new leishmanicidal agent.


