MEN 11270Selective B2 antagonist; analog of HOE 140 CAS# 235082-52-7 |
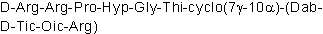
- NQDI 1
Catalog No.:BCC2404
CAS No.:175026-96-7
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Mdivi 1
Catalog No.:BCC2402
CAS No.:338967-87-6
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Cesium chloride
Catalog No.:BCC2399
CAS No.:7647-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 235082-52-7 | SDF | Download SDF |
| PubChem ID | 134812816 | Appearance | Powder |
| Formula | C60H90N20O11S | M.Wt | 1299.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 1 mg/ml in 30% acetonitrile / water | ||
| Sequence | RRPXGXXXXR (Modifications: Arg-1 = D-Arg, X-1 = Hyp, X-2 = Thi, X-3 = Dab, X-4 = D-Tic, X-5 = Oic, cyclized 7γ - 10α) | ||
| Chemical Name | (2S,4R)-1-[(2S)-1-[(2S)-2-[[(2R)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]-N-[2-[[(2R)-1-[[(1R,11S,14S)-14-[3-(diaminomethylideneamino)propyl]-2,12,15,20-tetraoxo-3,13,16,21-tetrazapentacyclo[19.8.0.03,11.04,9.023,28]nonacosa-23,25,27-trien-19-yl]amino]-1-oxo-3-thiophen-2-ylpropan-2-yl]amino]-2-oxoethyl]-4-hydroxypyrrolidine-2-carboxamide | ||
| SMILES | C1CCC2C(C1)CC3N2C(=O)C4CC5=CC=CC=C5CN4C(=O)C(CCNC(=O)C(NC3=O)CCCN=C(N)N)NC(=O)C(CC6=CC=CS6)NC(=O)CNC(=O)C7CC(CN7C(=O)C8CCCN8C(=O)C(CCCN=C(N)N)NC(=O)C(CCCN=C(N)N)N)O | ||
| Standard InChIKey | LPLBKEKLEUYDEJ-IEQIHQKBSA-N | ||
| Standard InChI | InChI=1S/C60H90N20O11S/c61-38(14-5-20-69-58(62)63)49(83)75-40(16-7-22-71-60(66)67)54(88)77-24-8-18-44(77)56(90)79-32-36(81)28-45(79)52(86)72-30-48(82)73-42(29-37-13-9-25-92-37)51(85)76-41-19-23-68-50(84)39(15-6-21-70-59(64)65)74-53(87)46-27-34-11-3-4-17-43(34)80(46)57(91)47-26-33-10-1-2-12-35(33)31-78(47)55(41)89/h1-2,9-10,12-13,25,34,36,38-47,81H,3-8,11,14-24,26-32,61H2,(H,68,84)(H,72,86)(H,73,82)(H,74,87)(H,75,83)(H,76,85)(H4,62,63,69)(H4,64,65,70)(H4,66,67,71)/t34?,36-,38-,39+,40+,41?,42-,43?,44+,45+,46+,47-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Peptide antagonist of the B2 bradykinin receptor (pKi = 10.3); conformationally constrained cyclized analog of HOE 140. Blocks hypotension and bronchoconstriction in vivo. Displays selectivity for B2 over 29 other receptors and ion channels (pIC50 < 5.5). |

MEN 11270 Dilution Calculator

MEN 11270 Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Axillaridine
Catalog No.:BCN2060
CAS No.:23506-96-9
- Peimine
Catalog No.:BCN1094
CAS No.:23496-41-5
- Hoechst 33258 analog 5
Catalog No.:BCC1627
CAS No.:23491-55-6
- Hoechst 33258 analog 2
Catalog No.:BCC1625
CAS No.:23491-54-5
- Hoechst 33342
Catalog No.:BCC1629
CAS No.:23491-52-3
- Hoechst 33258
Catalog No.:BCC1623
CAS No.:23491-45-4
- 2-Amino-5-mercapto-1,3,4-thiadiazole
Catalog No.:BCC8536
CAS No.:2349-67-9
- U 99194 maleate
Catalog No.:BCC7029
CAS No.:234757-41-6
- 2-Palmitoylglycerol
Catalog No.:BCC7289
CAS No.:23470-00-0
- trans-Khellactone
Catalog No.:BCN6920
CAS No.:23458-04-0
- Decursinol
Catalog No.:BCN2638
CAS No.:23458-02-8
- alpha-Spinasterone
Catalog No.:BCN5086
CAS No.:23455-44-9
- Humulon
Catalog No.:BCC8186
CAS No.:23510-81-8
- 8-Gingerol
Catalog No.:BCN5921
CAS No.:23513-08-8
- 6-Gingerol
Catalog No.:BCN1030
CAS No.:23513-14-6
- 10-Gingerol
Catalog No.:BCN5922
CAS No.:23513-15-7
- (-)-licarin A
Catalog No.:BCN5087
CAS No.:23518-30-1
- Vomifoliol
Catalog No.:BCN5088
CAS No.:23526-45-6
- 5-Aza-2'-deoxycytidine
Catalog No.:BCN2169
CAS No.:2353-33-5
- 8-Debenzoylpaeoniflorin
Catalog No.:BCC8787
CAS No.:23532-11-8
- Daunorubicin HCl
Catalog No.:BCC5083
CAS No.:23541-50-6
- Hoechst 33258 analog 3
Catalog No.:BCC1626
CAS No.:23554-98-5
- HOE 32020
Catalog No.:BCC1620
CAS No.:23554-99-6
- Hoechst 34580
Catalog No.:BCC1632
CAS No.:23555-00-2
Solid-phase synthesis of MEN 11270, a new cyclic peptide kinin B2 receptor antagonist.[Pubmed:11192241]
J Pept Sci. 2000 Dec;6(12):612-20.
An efficient synthesis of the cyclic decapeptide MEN 11270 [H-DArg1-Arg2 Pro3-Hyp4-Gly5-Thi6-Dab7-DTic8-Oic9-Arg10 c(7gamma - 10alpha)] was developed. Two three-dimensional orthogonal strategies were applied and compared: Fmoc/Tos/Boc (procedure A) and Fmoc/Pmc/Dde (procedure B). Both resulted in a 23-step strategy comprising the stepwise solid-phase chain assembly of the linear protected peptide, partial deprotection, solution-phase cyclization and final full deprotection. The stepwise assembly of the linear peptide was optimized by double coupling and acylation time prolongation for critical residues (Tic, Dab, Thi, Pro). O-(7-azabenzotriazol-1-yl)-N,N,N',N' tetramethyluronium (HATU) was preferred as coupling reagent for Dab. In the cyclization step, the partial racemization of Arg10 (31% using 1-ethyl-3-(3'-dimethyl-aminopropyl) carbodiimide/1-hydroxybenzotriazole (EDC/HOBt) as activation system) was reduced to 3% with HATU. The final deprotection was performed in the presence of dimethylsulfide (procedure A) and thiocresol (procedure B) as scavengers, to avoid the sulfation of Hyp side chain. The final compound and the main by-products were characterized by mass spectroscopy (MS), nuclear magnetic resonance (NMR) and racemization test. Procedure B produced operationally simpler and more efficient results than A (28% overall yield versus 4%).
MEN 11270, A novel selective constrained peptide antagonist with high affinity at the human B2 kinin receptor.[Pubmed:10336513]
J Pharmacol Exp Ther. 1999 Jun;289(3):1250-6.
We investigated the pharmacological profile of MEN 11270, or H-D-Arg-Arg-Pro-Hyp-Gly-Thi-c(Dab-DTic-Oic-Arg)c(7gamma-10 alpha), a conformationally constrained derivative of the B2 kinin receptor antagonist Icatibant. MEN 11270 bound with high-affinity to the B2 kinin receptor constitutively expressed by WI38 human fibroblasts, inhibiting 3H-bradykinin (BK) with a pKi value of 10.3 +/- 0.08 (n = 5). The rank order of affinity of several peptide and nonpeptide antagonists was also assessed: Icatibant (pKi = 10.6) approximately MEN 11270 (pKi = 10.3) approximately B9430 (pKi = 10.0) > B9858 (pKi = 8.0) > FR173657 (pKi = 7.6) > WIN64338 (pKi = 7.2) > Lys-[des-Arg9, Leu8]-BK (pKi < 6) > [des-Arg9,Leu8]-BK (pKi < 5). MEN 11270 showed a low affinity in inhibiting 3H-Lys-[des-Arg9]-BK binding at the human B1 kinin receptor constitutively expressed by the same cells (pKi 6.0 +/- 0.33; n = 3). MEN 11270 showed no binding affinity (pIC50 < 5.5) at 29 different receptors and ion channels. In the human umbilical vein contraction assay, MEN 11270, shifted the concentration-response curve to BK to the right in a concentration-dependent manner (pA2 8.14 +/- 0.22, n = 7). The Schild plot was linear (slope 0.95 +/- 0.11), consistent with a competitive antagonism. In the same bioassay, MEN 11270 (10 microM) did not affect the concentration-response curve to the B1 agonist Lys-[des-Arg9]-BK nor the contractile responses elicited by noradrenaline or serotonin. These findings indicate MEN 11270 as an antagonist at the human B2 kinin receptor, with potency and selectivity comparable to those of the linear peptide antagonist, supporting the hypothesis that a constrained C-terminal beta-turn conformation preserves a high affinity for the interaction of Icatibant with the B2 kinin receptor.
Interaction of linear and cyclic peptide antagonists at the human B(2) kinin receptor.[Pubmed:12182947]
Peptides. 2002 Aug;23(8):1457-63.
The ligand receptor interactions involving the C-terminal moiety of kinin B(2) receptor antagonists Icatibant (H-DArg-Arg-Pro-Hyp-Gly-Thi-Ser-Dtic-Oic-Arg-OH), MEN 11270 (H-DArg-Arg-Pro-Hyp-Gly-Thi-c(Dab-Dtic-Oic-Arg)c(7gamma-10alpha)) and a series of analogs modified in position 10 were investigated by radioligand-binding experiments at the wild type (WT) and at the Ser(111)Ala and Ser(111)Lys mutant human kinin B(2) receptors. Icatibant and [Lys(10)]-Icatibant maintained the same high affinity towards the three receptors. For Icatibant-NH(2), [Ala(10)]-Icatibant, MEN 11270 and [Glu(10)]-MEN 11270, the changes in affinity at the WT and Ser(111)Lys receptors indicated that the presence of a net positive or negative charge at the C-terminal moiety of these peptides caused a decrease in affinity to the WT receptor and that Ser(111) residue is in proximity of the side chain of residue 10. The changes in affinity measured with [desArg(10)]-Icatibant and [desArg(10)]-Icatibant-NH(2), moreover, confirmed that a C-terminal charge compensation between the positive charge of Arg(10) side chain and the C-terminal free carboxylic function favours a high affinity interaction.
Differences between peptide and nonpeptide B(2) bradykinin receptor antagonists in blocking bronchoconstriction and hypotension induced by bradykinin in anesthetized Guinea pigs.[Pubmed:11181940]
J Pharmacol Exp Ther. 2001 Mar;296(3):1051-7.
We have compared the in vivo activity of the bradykinin B(2) receptor peptide antagonists MEN 11270 and Icatibant versus the nonpeptide antagonist FR 173657, after intravenous (i.v.) and intratracheal (i.t.) administration, on the bradykinin (BK)-induced bronchoconstriction and hypotension in anesthetized guinea pigs. We have also assessed the affinity of these antagonists for B(2) receptors in guinea pig lung membranes by radioligand binding and the metabolic stability of peptide antagonists in guinea pig plasma and tissue homogenates. The i.v. administration of MEN 11270, Icatibant, or FR 173657 induced a dose-dependent (10-100 nmol/kg) inhibition of both hypotension and bronchoconstriction induced by bradykinin (10 nmol/kg i.v.). The inhibitory effect of MEN 11270 and Icatibant was comparable both in terms of potency and time course, whereas FR 173657 was less potent and shorter acting. After i.t. administration MEN 11270 and Icatibant (10-100 nmol/kg) dose dependently inhibited both bronchoconstriction and hypotension, whereas FR 173657 (10-100 nmol/kg) reduced bronchoconstriction without affecting hypotension. The antibronchoconstrictor effect of MEN 11270 was more prolonged than that of Icatibant and FR 173657, whereas no differences were found between the peptide antagonists in inhibiting hypotension. These findings indicated that, in vivo, the peptide antagonists are more potent and longer lasting than FR 173657 acting on bradykinin B(2) receptors in guinea pig airways and in the vascular system. The greater efficacy of the antagonists in blocking airway compared with vascular B(2) receptors after topical administration suggests that they can block airway B(2) receptors with little systemic effects.


