Prismatomeris tetrandra
Prismatomeris tetrandra
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Prismatomeris tetrandra
- Cat.No. Product Name CAS Number COA
-
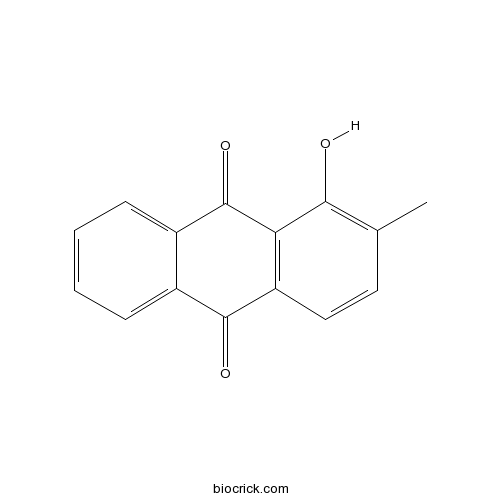
BCN6047
Rubiadin117-02-2
Instructions

-
BCN3478
1-Hydroxy-2-methylanthraquinone6268-09-3
Instructions
-
BCN4298
Rubiadin 1-methyl ether7460-43-7
Instructions

Hyaluronidase Inhibitory Activity of Pentacylic Triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, Synthesis and QSAR Study.[Pubmed: 26907251]
The mammalian hyaluronidase degrades hyaluronic acid by the cleavage of the β-1,4-glycosidic bond furnishing a tetrasaccharide molecule as the main product which is a highly angiogenic and potent inducer of inflammatory cytokines. Ursolic acid 1, isolated from Prismatomeris tetrandra, was identified as having the potential to develop inhibitors of hyaluronidase. A series of ursolic acid analogues were either synthesized via structure modification of ursolic acid 1 or commercially obtained. The evaluation of the inhibitory activity of these compounds on the hyaluronidase enzyme was conducted. Several structural, topological and quantum chemical descriptors for these compounds were calculated using semi empirical quantum chemical methods. A quantitative structure activity relationship study (QSAR) was performed to correlate these descriptors with the hyaluronidase inhibitory activity. The statistical characteristics provided by the best multi linear model (BML) (R² = 0.9717, R²cv = 0.9506) indicated satisfactory stability and predictive ability of the developed model. The in silico molecular docking study which was used to determine the binding interactions revealed that the ursolic acid analog 22 had a strong affinity towards human hyaluronidase.
Anthraquinones from the roots of Prismatomeris tetrandra.[Pubmed: 20839629]
A new anthraquinone compound, 1,3-dihydroxy-6-methoxy-2-methoxymethyl-9,10-anthraquinone (1), along with four known analogues (2-5) were isolated from the roots of Prismatomeris tetrandra. Their structures were elucidated on the basis of spectroscopic analysis. Among these compounds, lucidin omega-methyl ether (4) and lucidin omega-ethyl ether (5) were isolated from this genus for the first time. Compound 1 showed weak cytotoxic activity against A549 and LAC cell lines.
Determination of the absolute configurations of pharmacological natural products via density functional theory calculations of vibrational circular dichroism: the new cytotoxic iridoid prismatomerin.[Pubmed: 17784774]
A new highly cytotoxic iridoid has very recently been isolated from Prismatomeris tetrandra and shown to have the structure 3, similar to that of the iridoid oruwacin, 2. We report the determination of the absolute configuration (AC) of the new iridoid, prismatomerin, using vibrational circular dichroism (VCD) spectroscopy. The VCD spectrum of the acetate derivative of 3, 4, is analyzed using the Stephens theory of VCD and density functional theory (DFT). The AC of the naturally occurring 3 is shown to be 1R,5S,8S,9S,10S, identical to that of the naturally occurring iridoid plumericin, 1, also determined using VCD spectroscopy. The [alpha]D values of the natural products 3 and 1 are negative and positive, respectively. Since the ACs of 3 and 1 are identical, it follows that the AC of 3 cannot be correctly determined by empirical comparison of the signs of the [alpha]D values of 3 and 1.
Prismatomerin, a new iridoid from Prismatomeris tetrandra. Structure elucidation, determination of absolute configuration, and cytotoxicity.[Pubmed: 17665951]
A new complex iridoid, prismatomerin (1), has been isolated from the leaves of Prismatomeris tetrandra, together with the known glucoside gaertneroside (4). The structures of 1 and 4 were determined by spectroscopic analysis, notably 2D NMR techniques. The (1R,5S,8S,9S,10S)-(-) absolute configuration of prismatomerin (1) was determined by comparison of the vibrational circular dichroism (VCD) spectrum calculated using density functional theory and the experimental VCD spectrum of the O-acetyl derivative 3. Prismatomerin (1) showed remarkable antitumor activity and also interfered with mitotic spindle formation.
[Chemical constituents from root of Prismatomeris tetrandra].[Pubmed: 16468371]
To study the chemical constituents from root of Prismatomeris tetrandra.
Anthraquinones from the roots of Prismatomeris tetrandra.[Pubmed: 16204995]
Three new anthraquinones, 1-hydroxy-2,3-dimethoxy-7-methyl-9,10-anthraquinone, 1,3-dihydroxy-5,6-dimethoxy-2-methyl-9,10-anthraquinone, and 3-hydroxy-1,5,6-trimethoxy-2-methyl-9,10-anthraquinone, along with five known anthraquinones were isolated from the roots of Prismatomeris tetrandra. Their structures were determined on the basis of spectroscopic data.


