Schisandra propinqua
Schisandra propinqua
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Schisandra propinqua
- Cat.No. Product Name CAS Number COA
-
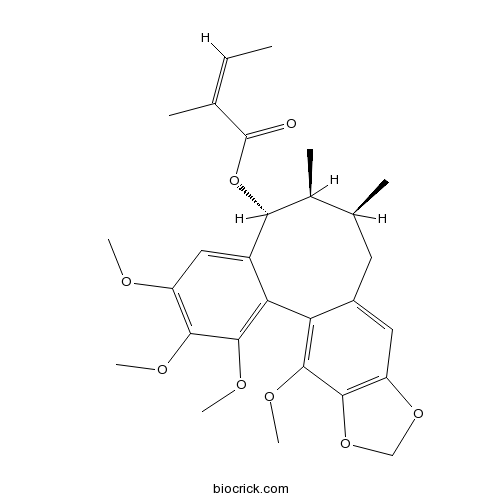
BCN7361
Angeloylgomisin O83864-69-1
Instructions
Five new schinortriterpenoids from Schisandra propinqua var. propinqua.[Pubmed: 29454022]
Five new schinortriterpenoids, propinqtrilactones A and B (1 and 2) with rare lancischiartane scaffold, and propindilactones V-X (3-5), were isolated from the stems and leaves of Schisandra propinqua var. propinqua. Their structures were elucidated on the basis of comprehensive spectroscopic and mass spectrometric analysis. The absolute configurations of 1-5 were determined by CD methods, X-ray diffraction analysis and theoretical calculations. 4 was tested for its cytotoxic activities against five human tumor cell lines.
[Rapidly identify chemical constituents of Schisandra propinqua by UPLC-Q-Tof/MSE].[Pubmed: 29271166]
This study was to identify the chemical constituents of Schisandra propinqua, one herbal medicine of Yi nationality in China by using ultra performance liquid chromatography-quadrupole-time of flight-mass spectrometry (UPLC-Q-TOF/MSE). Acetonitrile- water containing 0.1% formic acid was used as the mobile phase for gradient elution. Data were collected under ESI negative mode and ESI positive mode, and then screened and verified by the software of UNIFI and Masslynx4.1. Based on the accurate mass, fragment ions, neutral losses, mass error, retention time, reference substance, isotope information, the intensity of fragments, as well as the previous reports, the known compounds were validated and identified. The chemical structures of the unknown components were identified according to exact molecular weight, MS fragment, chromatographic retention behavior, and characteristic fragments of known congener compounds. A total of 68 chemical components were identified from S. propinqua, including 3 flavonoids, 10 flavanols, 34 lignans compounds (including 20 dibenzocyclooctene lignans), 4 triterpenoids, 17 organic acid and other compounds. 37 compounds of them were found in S. propinqua for the first time, and one potential compound needed to be identified.
Two New Compounds from Schisandra propinqua var. propinqua.[Pubmed: 28470483]
Schisanpropinoic acid (1), a new bergamotane sesquiterpenoid, and schisanpropinin (2), a new tetrahydrofuran lignan with a rare epoxyethane unit, were identified from the stems and leaves of Schisandra propinqua var. propinqua. Their structures were determined based on comprehensive spectroscopic and mass spectrometric analysis. The absolute configuration of 1 was determined by X-ray analysis. Compounds 1 and 2 were tested for their cytotoxic activity against five human tumor cell lines.
Asymmetric Total Synthesis of Propindilactone G.[Pubmed: 26181605]
A concise total synthesis of (+)-propindilactone G, a nortriterpenoid isolated from the stems of Schisandra propinqua var. propinqua, has been achieved for the first time. The key steps of the synthesis include an asymmetric Diels-Alder reaction, a Pauson-Khand reaction, a Pd-catalyzed reductive hydrogenolysis reaction, and an oxidative heterocoupling reaction. These reactions enabled the synthesis of (+)-propindilactone G in only 20 steps. As a consequence of our synthetic studies, the structure of (+)-propindilactone G has been revised.
[Determination of lignans in four fruits of Schisandra genus in Qinling mountains].[Pubmed: 24417136]
To determine the content of four lignans in the fruits of Schisandra sphenanthera, Schisandra grandiflora, Schisandra rubriflora and Schisandra propinqua subsp. sinensis.
Three new arylnaphthalene lignans from Schisandra propinqua var. sinensis.[Pubmed: 22100376]
Three new arylnaphthalene lignans, named sinensisins A-C (1-3), together with three known compounds, were isolated from the aerial parts of Schisandra propinqua var. sinensis. Their structures were established by spectroscopic methods, and compound 1 exhibited weak anti-HIV-1 activity with an TI value of 6.7.
Schisanartane nortriterpenoids with diverse post-modifications from Schisandra propinqua.[Pubmed: 20681569]
Twenty-one highly oxygenated nortriterpenoids with a schisanartane skeleton were isolated from the stems of Schisandra propinqua var. propinqua. These nortriterpenoids featured a polycyclic framework composed of 7/8/5 consecutive carbocycles, which were organized by similar 5/5/7/5/7/5 rings A-F and varied oxygen-containing rings G and H. A biosynthetic classification of these compounds is proposed on the basis of their diverse post-modifications.
Bisnortriterpenoids possessing an 18-Nor-schiartane skeleton from Schisandra propinqua var. propinqua.[Pubmed: 20309795]
Six C (28) triterpenoids with an 18-nor-schiartane framework were isolated from the aerial parts of SCHISANDRA PROPINQUA var. PROPINQUA. The new compounds, propindilactones P-S ( 1- 4), were structurally characterized by extensive spectroscopic methods. New compounds 2 and 3, together with known wuweizidilactones B and H ( 5- 6), were evaluated for their anti-HBV activity.


