Angeloylgomisin OCAS# 83864-69-1 |
- Tigloylgomisin O
Catalog No.:BCN8880
CAS No.:130855-74-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83864-69-1 | SDF | Download SDF |
| PubChem ID | 91864462 | Appearance | Powder |
| Formula | C28H34O8 | M.Wt | 498.57 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
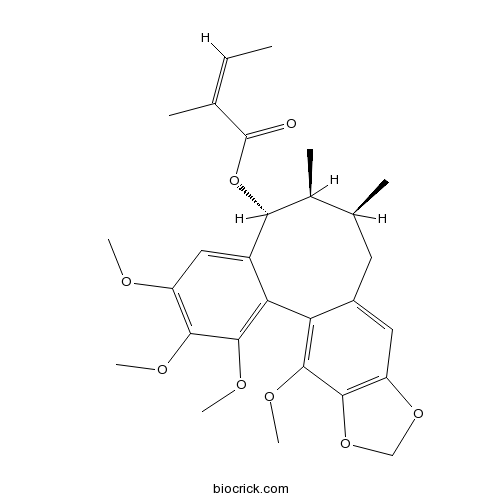
| SMILES | CC=C(C)C(=O)OC1C(C(CC2=CC3=C(C(=C2C4=C(C(=C(C=C14)OC)OC)OC)OC)OCO3)C)C | ||
| Standard InChIKey | PLKFSXFJGNZAER-XXDSNBTQSA-N | ||
| Standard InChI | InChI=1S/C28H34O8/c1-9-14(2)28(29)36-23-16(4)15(3)10-17-11-20-25(35-13-34-20)26(32-7)21(17)22-18(23)12-19(30-5)24(31-6)27(22)33-8/h9,11-12,15-16,23H,10,13H2,1-8H3/b14-9-/t15-,16-,23+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Angeloylgomisin O is a nartural product from Schisandra chinensis. |
| In vitro | The Antiproliferative Effects of Compounds Isolated from Schisandra chinensis.[Reference: WebLink]Korean Journal of Food Science & Technology, 2014, 46(6):665-670.We isolated twelve lignans and three terpenoids were isolated from the n-hexane fraction of Schisandra chinensis extract. |
| Structure Identification | Chemical & Pharmaceutical Bulletin, 1982, 30(9):3202-3206.The constituents of Schisandra chinensis Baill. XI. The structures of three new lignans, angeloylgomisin O, and angeloyl- and benzoylisogomisin O[Reference: WebLink]Three new dibenzocyclooctadiene lignans, Angeloylgomisin O (1), and angeloyl-(2) and benzoylisogomisin O (3) were isolated from the fruits of Schizandra chinensis BAILL. (Schizandraceae). Their absolute structures were elucidated by means of chemical and spectral studies. |

Angeloylgomisin O Dilution Calculator

Angeloylgomisin O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0057 mL | 10.0287 mL | 20.0574 mL | 40.1147 mL | 50.1434 mL |
| 5 mM | 0.4011 mL | 2.0057 mL | 4.0115 mL | 8.0229 mL | 10.0287 mL |
| 10 mM | 0.2006 mL | 1.0029 mL | 2.0057 mL | 4.0115 mL | 5.0143 mL |
| 50 mM | 0.0401 mL | 0.2006 mL | 0.4011 mL | 0.8023 mL | 1.0029 mL |
| 100 mM | 0.0201 mL | 0.1003 mL | 0.2006 mL | 0.4011 mL | 0.5014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4-Benzoyl 4'-methyldiphenyl sulfide
Catalog No.:BCC8694
CAS No.:83846-85-9
- 2-Ethylhexyl trans-4-methoxycinnamate
Catalog No.:BCN1333
CAS No.:83834-59-7
- Falecalcitriol
Catalog No.:BCC1570
CAS No.:83805-11-2
- Fmoc-Ser(Bzl)-OH
Catalog No.:BCC3542
CAS No.:83792-48-7
- Fmoc-Arg(Tos)-OH
Catalog No.:BCC3076
CAS No.:83792-47-6
- YM 244769
Catalog No.:BCC6222
CAS No.:837424-39-2
- WH-4-023
Catalog No.:BCC8051
CAS No.:837422-57-8
- Salvinorin A
Catalog No.:BCC5875
CAS No.:83729-01-5
- 3alpha-Acetoxy-20(29)-lupene-23,28-dioic acid
Catalog No.:BCN7508
CAS No.:83725-41-1
- Pomolic acid 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN1334
CAS No.:83725-24-0
- Ilexside I
Catalog No.:BCN3244
CAS No.:83725-19-3
- Dihydrosesamin
Catalog No.:BCN6616
CAS No.:83708-70-7
- Angeloylisogomisin O
Catalog No.:BCN4379
CAS No.:83864-70-4
- Obovatol
Catalog No.:BCN8265
CAS No.:83864-78-2
- Cetirizine
Catalog No.:BCC1469
CAS No.:83881-51-0
- Cetirizine DiHCl
Catalog No.:BCC4517
CAS No.:83881-52-1
- WIKI4
Catalog No.:BCC2455
CAS No.:838818-26-1
- TCS 2312
Catalog No.:BCC7541
CAS No.:838823-31-7
- Azithromycin
Catalog No.:BCC4385
CAS No.:83905-01-5
- Gramodendrine
Catalog No.:BCN2155
CAS No.:83905-67-3
- 12-Acetoxyabietic acid
Catalog No.:BCN4380
CAS No.:83905-81-1
- 13-Hydroxylabda-8(17),14-dien-18-oic acid
Catalog No.:BCN1332
CAS No.:83915-59-7
- Isogomisin O
Catalog No.:BCN4381
CAS No.:83916-76-1
- Mometasone furoate
Catalog No.:BCC4801
CAS No.:83919-23-7
Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts.[Pubmed:30486445]
Molecules. 2018 Nov 27;23(12). pii: molecules23123103.
Schisandra rubriflora is a dioecious plant of increasing importance due to its lignan composition, and therefore, possible therapeutic properties. The aim of the work was lignan profiling of fruits, leaves and shoots of female (F) and male (M) plants using UHPLC-MS/MS. Additionally, the anti-inflammatory activity of plant extracts and individual lignans was tested in vitro for the inhibition of 15-lipooxygenase (15-LOX), phospholipases A2 (sPLA(2)), cyclooxygenase 1 and 2 (COX-1; COX-2) enzyme activities. The extracts of fruits, leaves and shoots of the pharmacopoeial species, S. chinensis, were tested for comparison. Twenty-four lignans were monitored. Lignan contents in S. rubriflora fruit extracts amounted to 1055.65 mg/100 g DW and the dominant compounds included schisanhenol, aneloylgomisin H, schisantherin B, schisandrin A, gomisin O, Angeloylgomisin O and gomisin G. The content of lignan in leaf extracts was 853.33 (F) and 1106.80 (M) mg/100 g DW. Shoot extracts were poorer in lignans-559.97 (F) and 384.80 (M) mg/100 g DW. Schisantherin B, schisantherin A, 6-O-benzoylgomisin O and angeloylgomisin H were the dominant compounds in leaf and shoot extracts. The total content of detected lignans in S. chinensis fruit, leaf and shoot extracts was: 1686.95, 433.59 and 313.83 mg/100 g DW, respectively. Gomisin N, schisandrin A, schisandrin, gomisin D, schisantherin B, gomisin A, angeloylgomisin H and gomisin J were the dominant lignans in S. chinensis fruit extracts were. The results of anti-inflammatory assays revealed higher activity of S. rubriflora extracts. Individual lignans showed significant inhibitory activity against 15-LOX, COX-1 and COX-2 enzymes.
[Studies on chemical constituents of Schisandra propinqua (Wall.) Hook. f. et Thoms].[Pubmed:12776319]
Zhongguo Zhong Yao Za Zhi. 2001 Oct;26(10):694-7.
OBJECTIVE: To isolate and characterize compounds from the stems of Schisandra propinqua. METHOD: Extracting with solvent, isolating by column chromatography and identifying by the spectroscopic methods. RESULT: Six dibenzocyclooctadiene lignans were isolated and identified as tigloylgomisin P(1), Angeloylgomisin O(2), angeloylisogomisin O(3), kadsulignan L(4), (+/-) 5,8-epoxyl-6, 7-dimethyl-2',3',2",3"-dimethylenedioxy-4', 1"-dimethyl-1,2:3,4-dibenzo-1, 3-cyclooctadiene(5) and wuweizisu C(6). CONCLUSION: Compounds 4 and 5 were the first two dibenzocyclooctadiene lignans with an 6,9-epoxy bridge cycle discovered in the genus Schisandra. The others were originally isolated from S. propinqua.


