Isogomisin OCAS# 83916-76-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83916-76-1 | SDF | Download SDF |
| PubChem ID | 634476 | Appearance | Powder |
| Formula | C23H28O7 | M.Wt | 416.5 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
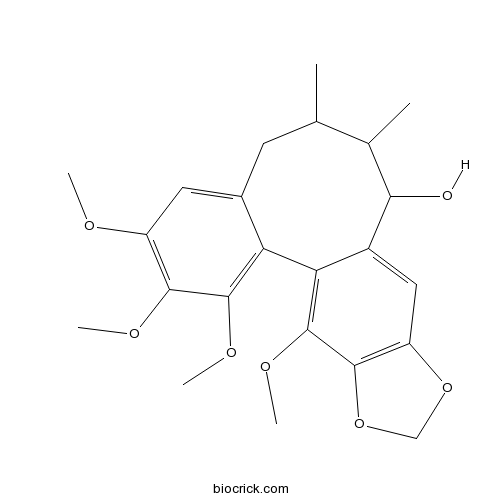
| Chemical Name | 3,4,5,19-tetramethoxy-9,10-dimethyl-15,17-dioxatetracyclo[10.7.0.02,7.014,18]nonadeca-1(19),2,4,6,12,14(18)-hexaen-11-ol | ||
| SMILES | CC1CC2=CC(=C(C(=C2C3=C(C4=C(C=C3C(C1C)O)OCO4)OC)OC)OC)OC | ||
| Standard InChIKey | YVMJUSKDPJGDHW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H28O7/c1-11-7-13-8-15(25-3)20(26-4)22(27-5)17(13)18-14(19(24)12(11)2)9-16-21(23(18)28-6)30-10-29-16/h8-9,11-12,19,24H,7,10H2,1-6H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| In vitro | Composition and biological activity of different extracts from Schisandra sphenanthera and Schisandra chinensis.[Pubmed: 17611932]Planta Med. 2007 Aug;73(10):1116-26.Plants of the Schisandraceae family contain a variety of pharmacologically active lignans like schizandrin, deoxyschizandrin, deangeloylgomisin B, gomisin A, gomisin O, gamma-schizandrin and Isogomisin O.
|
| Structure Identification | Chem Pharm Bull (Tokyo). 2007 Aug;55(8):1281-3.Lignans from Schisandra propinqua var. propinqua.[Pubmed: 17666862]
|

Isogomisin O Dilution Calculator

Isogomisin O Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.401 mL | 12.0048 mL | 24.0096 mL | 48.0192 mL | 60.024 mL |
| 5 mM | 0.4802 mL | 2.401 mL | 4.8019 mL | 9.6038 mL | 12.0048 mL |
| 10 mM | 0.2401 mL | 1.2005 mL | 2.401 mL | 4.8019 mL | 6.0024 mL |
| 50 mM | 0.048 mL | 0.2401 mL | 0.4802 mL | 0.9604 mL | 1.2005 mL |
| 100 mM | 0.024 mL | 0.12 mL | 0.2401 mL | 0.4802 mL | 0.6002 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 13-Hydroxylabda-8(17),14-dien-18-oic acid
Catalog No.:BCN1332
CAS No.:83915-59-7
- 12-Acetoxyabietic acid
Catalog No.:BCN4380
CAS No.:83905-81-1
- Gramodendrine
Catalog No.:BCN2155
CAS No.:83905-67-3
- Azithromycin
Catalog No.:BCC4385
CAS No.:83905-01-5
- TCS 2312
Catalog No.:BCC7541
CAS No.:838823-31-7
- WIKI4
Catalog No.:BCC2455
CAS No.:838818-26-1
- Cetirizine DiHCl
Catalog No.:BCC4517
CAS No.:83881-52-1
- Cetirizine
Catalog No.:BCC1469
CAS No.:83881-51-0
- Obovatol
Catalog No.:BCN8265
CAS No.:83864-78-2
- Angeloylisogomisin O
Catalog No.:BCN4379
CAS No.:83864-70-4
- Angeloylgomisin O
Catalog No.:BCN7361
CAS No.:83864-69-1
- 4-Benzoyl 4'-methyldiphenyl sulfide
Catalog No.:BCC8694
CAS No.:83846-85-9
- Mometasone furoate
Catalog No.:BCC4801
CAS No.:83919-23-7
- Flavidin
Catalog No.:BCN6438
CAS No.:83924-98-5
- Flavidinin
Catalog No.:BCN3599
CAS No.:83925-00-2
- 4-Epicommunic acid
Catalog No.:BCN4382
CAS No.:83945-57-7
- GNF-7
Catalog No.:BCC6529
CAS No.:839706-07-9
- Pluripotin
Catalog No.:BCC6178
CAS No.:839707-37-8
- 2,7-Dimethyl-1,4-dihydroxynaphthalene 1-O-glucoside
Catalog No.:BCN7611
CAS No.:839711-70-5
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
- Rutaecarpine
Catalog No.:BCN4385
CAS No.:84-26-4
- Ophiohayatone C
Catalog No.:BCN3608
CAS No.:84-33-3
- Syrosingopine
Catalog No.:BCN5365
CAS No.:84-36-6
Lignans from Schisandra propinqua var. propinqua.[Pubmed:17666862]
Chem Pharm Bull (Tokyo). 2007 Aug;55(8):1281-3.
Two new dibenzocyclooctadiene lignans angeloyl-(+)-gomisin K(3) (1) and methylIsogomisin O (2), together with six known ones, Isogomisin O, angeloylIsogomisin O, gomisin O, angeloygomisin O, benzoylgomisin O, epigomisin O, and four 1,4-bis(phenyl)-2,3-dimethylbutane type lignans, pregomisin, meso-dihydroguaiaretic acid, isoanwulignan, and sphenanlignan were isolated from the aerial parts of Schisandra propinqua var. propinqua. The structures of 1 and 2 were elucidated by spectroscopic methods including extensive 1D- and 2D-NMR techniques.
Composition and biological activity of different extracts from Schisandra sphenanthera and Schisandra chinensis.[Pubmed:17611932]
Planta Med. 2007 Aug;73(10):1116-26.
Plants of the Schisandraceae family contain a variety of pharmacologically active lignans like schizandrin, deoxyschizandrin, deangeloylgomisin B, gomisin A, gomisin O, gamma-schizandrin and Isogomisin O. Here we have compared the composition of different polar and non-polar extracts of Schisandra sphenanthera and Schisandra chinensis. We also have screened the extracts for antiproliferative and anti-inflammatory effects in different cell-based and cell-free assays. Extracts produced with the non-polar solvents CO(2), hexane and CO(2)/5% ethanol had a similar composition. In contrast, polar extraction with ethanol provided a considerably higher yield, but a lower content of volatiles and lignans in comparison to the non-polar extracts. The proliferation of the epidermal cell lines HaCaT and A431 was dose-dependently inhibited by both the Schisandra sphenanthera and Schisandra chinensis extracts, the non-polar extracts being superior to the polar ones. The non-polar Schisandra sphenanthera extract was the most active with a half-maximal inhibitory concentration of 20 microg/mL. In a cell-free enzyme inhibition assay with recombinant cyclooxygenase-2 (COX-2) the non-polar Schisandra sphenanthera extract dose-dependently inhibited COX-2 catalysed prostaglandin (PG) production (IC(50) = 0.2 microg/mL). It also reduced the ultraviolet-B (UVB)-induced PGE (2) production (IC(50) = 4 microg/mL) and COX-2 expression in HaCaT keratinocytes. We conclude that non-polar SChisandra extracts obtained by CO(2) extraction might be useful in the prevention and treatment of hyperproliferative and inflammatory skin diseases.


