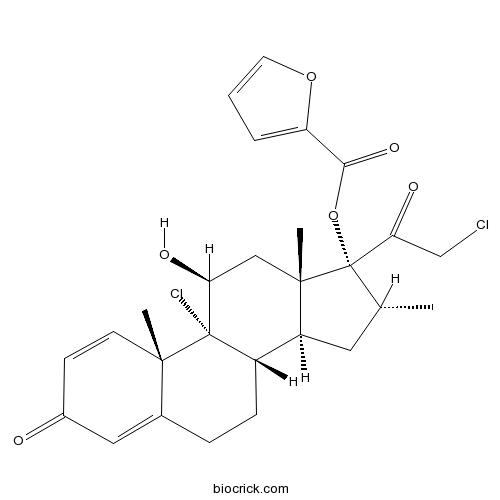
Mometasone furoateSynthetic corticosteroid; anti-inflammatory agent CAS# 83919-23-7 |
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- LAQ824 (NVP-LAQ824,Dacinostat)
Catalog No.:BCC2160
CAS No.:404951-53-7
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
- TC-H 106
Catalog No.:BCC2426
CAS No.:937039-45-7
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83919-23-7 | SDF | Download SDF |
| PubChem ID | 441336 | Appearance | Powder |
| Formula | C27H30Cl2O6 | M.Wt | 521.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (95.89 mM; Need ultrasonic) | ||
| Chemical Name | [(8S,9R,10S,11S,13S,14S,16R,17R)-9-chloro-17-(2-chloroacetyl)-11-hydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] furan-2-carboxylate | ||
| SMILES | CC1CC2C3CCC4=CC(=O)C=CC4(C3(C(CC2(C1(C(=O)CCl)OC(=O)C5=CC=CO5)C)O)Cl)C | ||
| Standard InChIKey | WOFMFGQZHJDGCX-ZULDAHANSA-N | ||
| Standard InChI | InChI=1S/C27H30Cl2O6/c1-15-11-19-18-7-6-16-12-17(30)8-9-24(16,2)26(18,29)21(31)13-25(19,3)27(15,22(32)14-28)35-23(33)20-5-4-10-34-20/h4-5,8-10,12,15,18-19,21,31H,6-7,11,13-14H2,1-3H3/t15-,18+,19+,21+,24+,25+,26+,27+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent synthetic corticosteroid; displays agonist activity at glucocorticoid and progesterone receptors. Exhibits anti-inflammatory effects. |

Mometasone furoate Dilution Calculator

Mometasone furoate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9178 mL | 9.589 mL | 19.178 mL | 38.3561 mL | 47.9451 mL |
| 5 mM | 0.3836 mL | 1.9178 mL | 3.8356 mL | 7.6712 mL | 9.589 mL |
| 10 mM | 0.1918 mL | 0.9589 mL | 1.9178 mL | 3.8356 mL | 4.7945 mL |
| 50 mM | 0.0384 mL | 0.1918 mL | 0.3836 mL | 0.7671 mL | 0.9589 mL |
| 100 mM | 0.0192 mL | 0.0959 mL | 0.1918 mL | 0.3836 mL | 0.4795 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mometasone furoate
- Isogomisin O
Catalog No.:BCN4381
CAS No.:83916-76-1
- 13-Hydroxylabda-8(17),14-dien-18-oic acid
Catalog No.:BCN1332
CAS No.:83915-59-7
- 12-Acetoxyabietic acid
Catalog No.:BCN4380
CAS No.:83905-81-1
- Gramodendrine
Catalog No.:BCN2155
CAS No.:83905-67-3
- Azithromycin
Catalog No.:BCC4385
CAS No.:83905-01-5
- TCS 2312
Catalog No.:BCC7541
CAS No.:838823-31-7
- WIKI4
Catalog No.:BCC2455
CAS No.:838818-26-1
- Cetirizine DiHCl
Catalog No.:BCC4517
CAS No.:83881-52-1
- Cetirizine
Catalog No.:BCC1469
CAS No.:83881-51-0
- Obovatol
Catalog No.:BCN8265
CAS No.:83864-78-2
- Angeloylisogomisin O
Catalog No.:BCN4379
CAS No.:83864-70-4
- Angeloylgomisin O
Catalog No.:BCN7361
CAS No.:83864-69-1
- Flavidin
Catalog No.:BCN6438
CAS No.:83924-98-5
- Flavidinin
Catalog No.:BCN3599
CAS No.:83925-00-2
- 4-Epicommunic acid
Catalog No.:BCN4382
CAS No.:83945-57-7
- GNF-7
Catalog No.:BCC6529
CAS No.:839706-07-9
- Pluripotin
Catalog No.:BCC6178
CAS No.:839707-37-8
- 2,7-Dimethyl-1,4-dihydroxynaphthalene 1-O-glucoside
Catalog No.:BCN7611
CAS No.:839711-70-5
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
- Rutaecarpine
Catalog No.:BCN4385
CAS No.:84-26-4
- Ophiohayatone C
Catalog No.:BCN3608
CAS No.:84-33-3
- Syrosingopine
Catalog No.:BCN5365
CAS No.:84-36-6
- Stylopine
Catalog No.:BCN3715
CAS No.:84-39-9
Formulation and Evaluation of Thermoreversible In Situ Nasal Gels Containing Mometasone Furoate for Allergic Rhinitis.[Pubmed:28281209]
AAPS PharmSciTech. 2017 Oct;18(7):2673-2682.
The purpose of the present work was to develop a mucoadhesive thermoreversible nasal gel with a tailored gelling temperature to provide the prolonged contact between Mometasone furoate and the nasal mucosa and in order to prevent drainage of the formulation. For this purpose, in situ gel containing a thermogelling polymer poloxamer 407 (Pluronic(R) F-127) and a mucoadhesive polymer Carbopol(R) 974P NF was prepared. In this content, formulations were designed to have gelation temperature below 34 degrees C to obtain gelation at intranasal cavity. Evaluation of the prepared in situ gels was carried out by the determination of sol-gel transition temperature, rheological and mechanical characteristics, mucoadhesion strength, drug content, physicochemical stability, in vitro release profiles, and ex vivo permeation across sheep nasal mucosa of formulations. Consequently, the in situ gel (CP5) which had favorable gelation temperature (30.1 +/- 0.24 degrees C), rheological and mechanical characteristics, in vitro release profile (T%100 180 min), and mucoadhesion strength (0.289 +/- 0.0069 mJ) was developed. Consequently, the in situ gel system has been concluded as a promising approach in order to improve the therapeutic effects of intranasal Mometasone furoate administration.
Combination of mometasone furoate and oxymetazoline for the treatment of adenoid hypertrophy concomitant with allergic rhinitis: A randomized controlled trial.[Pubmed:28098165]
Sci Rep. 2017 Jan 18;7:40425.
In the clinic, approximately 30% of children with adenoid hypertrophy (AH) concomitant with allergic rhinitis (AR) report poor responses to intranasal steroids. To determine whether the combination of Mometasone furoate (MF) and oxymetazoline (OXY) is more effective than either agent alone, we performed a two-stage, parallel, randomized, double-blind, double-dummy, clinical trial with 240 AH children with concomitant perennial AR. During the first stage, all children were randomly assigned to the MF or control group for six weeks of treatment. During the second stage, the non-responders from stage one were randomly assigned to 4 groups for 8 weeks of treatment that involved receiving the following treatments: MF/OXY, MF/placebo, placebo/OXY, or placebo/placebo. During the first stage of treatment, 39% of the responders treated with MF achieved greater reductions in total and individual symptom scores than did those on placebo. During the second stage of treatment, the nasal congestion scores of the MF/OXY group significantly decreased. The adenoid/choana ratio of the MF/OXY-treated group decreased and the nasal volume increased significantly. Our results suggest that the combination of OXY and MF is effective and safe for the treatment of AH children with concomitant AR and has a rapid onset of action.
Mometasone Furoate Suppresses PMA-Induced MUC-5AC and MUC-2 Production in Human Airway Epithelial Cells.[Pubmed:28119748]
Tuberc Respir Dis (Seoul). 2017 Jan;80(1):60-68.
BACKGROUND: Mucus hypersecretion from airway epithelium is a characteristic feature of airway inflammatory diseases. Tumor necrosis factor alpha (TNF-alpha) regulates mucin synthesis. Glucocorticoids including mometasone fuorate (MF) have been used to attenuate airway inflammation. However, effects of MF on mucin production have not been reported. METHODS: Effects of MF and budesonide (BUD) on the phorbol-12-myristate-13-acetate (PMA)-induction of mucin and TNF-alpha in human airway epithelial cells (NCI-H292) were investigated in the present study. Confluent NCI-H292 cells were pretreated with PMA (200 nM) for 2 hours. Subsequently, the cells were stimulated with MF (1-500 ng/mL) or BUD (21.5 ng/mL) for 8 hours. Dexamethasone (1 microg/mL) was used as the positive control. Real-time polymerase chain reaction was used to determine MUC2 and MUC5AC mRNA levels. The level of total mucin, MUC2, MUC5AC, and TNF-alpha in culture supernatants were measured using enzyme-linked immunosorbent assay. RESULTS: MF and BUD significantly suppressed MUC2 and MUC5AC gene expression in PMA-stimulated NCI-H292 cells. The inhibitory effects of the two steroid drugs were also observed in the production of total mucin, MUC2 and MUC5AC proteins, and TNF-alpha. CONCLUSION: Our findings demonstrated that MF and BUD attenuated mucin and TNF-alpha production in PMA-induced human airway epithelial cells.
Dissociation of local anti-inflammatory effect and systemic effects of mometasone furoate in mice.[Pubmed:19874229]
Immunopharmacol Immunotoxicol. 2009;31(4):601-6.
Mometasone furoate (MF) is a topical glucocorticoid used for atopic dermatitis, allergic rhinitis, and bronchial asthma. To elucidate the usefulness of MF, the dissociation between local anti-inflammatory effects and systemic effects of MF was compared with that of beclomethasone 17,21-dipropionate (BDP). MF was more potent than BDP in croton oil-induced ear edema tests in mice. Oral systemic effects of MF were inversely lower than that of BDP on thymolysis, plasma corticosterone lowering, and suppression of body weight gain in mice. These results indicate that MF has a higher therapeutic index and superior clinical usefulness as a topical glucocorticoid compared to BDP.
Mometasone furoate is a less specific glucocorticoid than fluticasone propionate.[Pubmed:12503693]
Eur Respir J. 2002 Dec;20(6):1386-92.
Fluticasone propionate (FP) and Mometasone furoate (MF) are potent synthetic corticosteroids that are widely used as anti-inflammatory agents to treat respiratory diseases. As part of the assessment of the potential for side-effects associated with their use, their activities, not only at the glucocorticoid receptor (GR) but also at the other members of the steroid nuclear receptor family, have been compared. Cell-based functional systems were established to measure different aspects of GR function, as well as the activity at all the other steroid nuclear receptors. The effects of MF and FP on the GR were potent and indistinguishable. Neither corticosteroid showed any activity at the oestrogen receptor, while both were weak antagonists of the androgen receptor. FP was a relatively weak agonist of the progesterone receptor but MF was a very potent agonist of the progesterone receptor, giving activity at similar concentrations to those that stimulate the GR (concentration generating 50% maximal effect (EC50)=50 pM). Moreover, while FP was a weak antagonist of the mineralocorticoid receptor (concentration generating 50% maximal inhibitory effect=80 nM), MF displayed potent partial agonist activity (EC50=3 nM, 30%). Mometasone furoate is considerably less specific for the glucocorticoid receptor than fluticasone propionate, showing significant activity at other nuclear steroid receptors.


