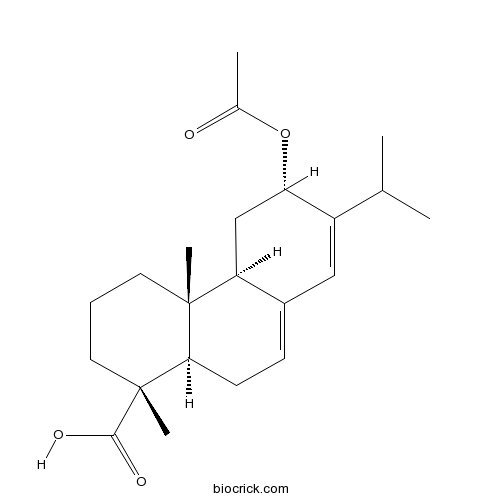
12-Acetoxyabietic acidCAS# 83905-81-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83905-81-1 | SDF | Download SDF |
| PubChem ID | 91895314 | Appearance | Powder |
| Formula | C22H32O4 | M.Wt | 360.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,4aR,4bR,6S,10aR)-6-acetyloxy-1,4a-dimethyl-7-propan-2-yl-2,3,4,4b,5,6,10,10a-octahydrophenanthrene-1-carboxylic acid | ||
| SMILES | CC(C)C1=CC2=CCC3C(C2CC1OC(=O)C)(CCCC3(C)C(=O)O)C | ||
| Standard InChIKey | FSSCSAJMAPLBRB-JIWOIOHBSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

12-Acetoxyabietic acid Dilution Calculator

12-Acetoxyabietic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7739 mL | 13.8696 mL | 27.7393 mL | 55.4785 mL | 69.3481 mL |
| 5 mM | 0.5548 mL | 2.7739 mL | 5.5479 mL | 11.0957 mL | 13.8696 mL |
| 10 mM | 0.2774 mL | 1.387 mL | 2.7739 mL | 5.5479 mL | 6.9348 mL |
| 50 mM | 0.0555 mL | 0.2774 mL | 0.5548 mL | 1.1096 mL | 1.387 mL |
| 100 mM | 0.0277 mL | 0.1387 mL | 0.2774 mL | 0.5548 mL | 0.6935 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gramodendrine
Catalog No.:BCN2155
CAS No.:83905-67-3
- Azithromycin
Catalog No.:BCC4385
CAS No.:83905-01-5
- TCS 2312
Catalog No.:BCC7541
CAS No.:838823-31-7
- WIKI4
Catalog No.:BCC2455
CAS No.:838818-26-1
- Cetirizine DiHCl
Catalog No.:BCC4517
CAS No.:83881-52-1
- Cetirizine
Catalog No.:BCC1469
CAS No.:83881-51-0
- Obovatol
Catalog No.:BCN8265
CAS No.:83864-78-2
- Angeloylisogomisin O
Catalog No.:BCN4379
CAS No.:83864-70-4
- Angeloylgomisin O
Catalog No.:BCN7361
CAS No.:83864-69-1
- 4-Benzoyl 4'-methyldiphenyl sulfide
Catalog No.:BCC8694
CAS No.:83846-85-9
- 2-Ethylhexyl trans-4-methoxycinnamate
Catalog No.:BCN1333
CAS No.:83834-59-7
- Falecalcitriol
Catalog No.:BCC1570
CAS No.:83805-11-2
- 13-Hydroxylabda-8(17),14-dien-18-oic acid
Catalog No.:BCN1332
CAS No.:83915-59-7
- Isogomisin O
Catalog No.:BCN4381
CAS No.:83916-76-1
- Mometasone furoate
Catalog No.:BCC4801
CAS No.:83919-23-7
- Flavidin
Catalog No.:BCN6438
CAS No.:83924-98-5
- Flavidinin
Catalog No.:BCN3599
CAS No.:83925-00-2
- 4-Epicommunic acid
Catalog No.:BCN4382
CAS No.:83945-57-7
- GNF-7
Catalog No.:BCC6529
CAS No.:839706-07-9
- Pluripotin
Catalog No.:BCC6178
CAS No.:839707-37-8
- 2,7-Dimethyl-1,4-dihydroxynaphthalene 1-O-glucoside
Catalog No.:BCN7611
CAS No.:839711-70-5
- Cariprazine
Catalog No.:BCC1453
CAS No.:839712-12-8
- Hexestrol
Catalog No.:BCC4484
CAS No.:84-16-2
- Rutaecarpine
Catalog No.:BCN4385
CAS No.:84-26-4
Effect of calcination temperature of a copper ferrite synthesized by a sol-gel method on its structural characteristics and performance as Fenton catalyst to remove gallic acid from water.[Pubmed:29024859]
J Colloid Interface Sci. 2018 Feb 1;511:193-202.
A copper ferrite synthesized by a sol-gel combustion method was calcined at different temperatures up to 800 degrees C, determining changes in its structural characteristics and magnetic measurements and studying its catalytic performance in gallic acid removal by Fenton reaction. The main objective was to study the effect of the calcination temperature of copper ferrite on its crystalline phase formation and transformation, activity and metal ion leaching. The cubic-to-tetragonal transformation of the spinel occurred via its reaction with the CuO phase, displacing Fe(3+) ions in B (octahedral) sites out of the spinel structure by the following reaction: 2Fe(3+)B+3CuO-->Fe2O3+3Cu(2+)B. The catalysts showed superparamagnetic or substantial superparamagnetic behaviour. At higher calcination temperatures, catalyst activity was lower, and Cu ion leaching was markedly decreased. There was no Fe ion leaching with any catalyst. The as-prepared catalyst showed better catalytic performance than a commercial copper ferrite. Leached Cu ions acted as homogeneous catalysts, and their contribution to the overall removal mechanism was examined. Cu2O present in the as-prepared catalysts made only a small contribution to their activity. Finally, the reutilization of various catalysts was studied by performing different catalytic cycles.
Biochemical, biological and molecular characterization of an L-Amino acid oxidase (LAAO) purified from Bothrops pictus Peruvian snake venom.[Pubmed:29024770]
Toxicon. 2017 Dec 1;139:74-86.
An L-amino acid oxidase from Peruvian Bothrops pictus (Bpic-LAAO) snake venom was purified using a combination of size-exclusion and ion-exchange chromatography. Bpic-LAAO is a homodimeric glycosylated flavoprotein with molecular mass of approximately 65 kDa under reducing conditions and approximately 132 kDa in its native form as analyzed by SDS-PAGE and gel filtration chromatography, respectively. N-terminal amino acid sequencing showed highly conserved residues in a glutamine-rich motif related to binding substrate. The enzyme exhibited optimal activity towards L-Leu at pH 8.5, and like other reported SV-LAAOs, it is stable until 55 degrees C. Kinetic studies showed that the cations Ca(2+), Mg(2+) and Mn(2+) did not alter Bpic-LAAO activity; however, Zn(2+) is an inhibitor. Some reagents such as beta-mercaptoethanol, glutathione and iodoacetate had inhibitory effect on Bpic-LAAO activity, but PMSF, EDTA and glutamic acid did not affect its activity. Regarding the biological activities of Bpic-LAAO, this enzyme induced edema in mice (MED = 7.8 mug), and inhibited human platelet aggregation induced by ADP in a dose-dependent manner and showed antibacterial activity on Gram (+) and Gram (-) bacteria. Bpic-LAAO cDNA of 1494 bp codified a mature protein with 487 amino acid residues comprising a signal peptide of 11 amino acids. Finally, the phylogenetic tree obtained with other sequences of LAAOs, evidenced its similarity to other homologous enzymes, showing two well-established monophyletic groups in Viperidae and Elapidae families. Bpic-LAAO is evolutively close related to LAAOs from B. jararacussu, B. moojeni and B. atrox, and together with the LAAO from B. pauloensis, form a well-defined cluster of the Bothrops genus.
Association between serum uric acid level and renal arteriolar hyalinization in individuals without chronic kidney disease.[Pubmed:29024864]
Atherosclerosis. 2017 Nov;266:121-127.
BACKGROUND AND AIMS: Recent studies have reported an association between serum uric acid (SUA) and renal arteriolar changes in patients with chronic kidney disease (CKD). However, the association in individuals without CKD remains unclear. In this study, we investigated the relationship between SUA and renal arteriolar lesions in individuals without CKD from our living kidney donor cohort. METHODS: Between January 2006 and May 2016, 393 living kidney donors underwent "time-zero" biopsy at Kyushu University Hospital. Patients were divided into sex-specific quartiles of SUA before donation: Q1, Q2, Q3, and Q4 (male: <5.2,5.2-5.8,5.9-6.4, and >/=6.5 mg/dL, female: <3.8,3.8-4.3,4.4-5.0, and >/=5.1 mg/dL). Renal arteriolar hyalinization and wall thickening were assessed using a semiquantitative grading system. Predictive performance was compared between models with and without SUA by calculating the net reclassification improvement (NRI). RESULTS: In total, 158 (40.2%) patients had arteriolar hyalinization, and 148 (37.6%) had wall thickening. High SUA was significantly associated with arteriolar hyalinization in multivariable logistic analysis (odds ratio [OR] per 1-mg/dL increase in SUA, 1.24; 95% confidence interval [CI], 1.00-1.53; p = 0.048. OR for Q4 vs. Q2, 2.22; 95% CI, 1.17-4.21; p = 0.01). We found no association between SUA and wall thickening. When SUA was incorporated into a predictive model with conventional atherosclerotic factors, the NRI was 0.21 (p = 0.04). CONCLUSIONS: High SUA was an independent risk factor for arteriolar hyalinization in individuals without CKD. SUA provided additional predictive value beyond conventional atherosclerotic factors in predicting arteriolar hyalinization.
Dimerization and oxidation of tryptophan in UV-A photolysis sensitized by kynurenic acid.[Pubmed:29024806]
Free Radic Biol Med. 2017 Dec;113:372-384.
Photoinduced generation of radicals in the eye lens may play an important role in the modification of proteins leading to their coloration, aggregation, and insolubilization. The radicals can be formed via the reactions of photoexcited endogenous chromophores of the human lens with lens proteins, in particular with tryptophan residues. In the present work we studied the reactions induced by UV-A (315-400nm) light between kynurenic acid (KNA), an effective photosensitizer present in the human lens, and N-acetyl-L-tryptophan (NTrpH) under aerobic and anaerobic conditions. Our results show that the reaction mechanism strongly depends on the presence of oxygen in solution. Under aerobic conditions, the generation of singlet oxygen is the major channel of the effective NTrpH oxidation. In argon-bubbled solutions, the quenching of triplet KNA by NTrpH results in the formation of KNA(*)(-) and NTrp(*) radicals. Under laser pulse irradiation, when the radical concentration is high, the main pathway of the radical decay is the back electron transfer with the restoration of initial reagents. Other reactions include (i) the radical combination yielding NTrp dimers and (ii) the oxygen atom transfer from KNA(*)(-) to NTrp(*) with the formation of oxidized NTrp species and deoxygenated KNA products. In continuous-wave photolysis, even trace amounts of molecular oxygen are sufficient to oxidize the majority of KNA(*)(-) radicals with the rate constant of (2.0 +/- 0.2) x 10(9)M(-1)s(-1), leading to the restoration of KNA and the formation of superoxide radical O2(*)(-). The latter reacts with NTrp(*) via either the radical combination to form oxidized NTrp (minor pathway), or the electron transfer to restore NTrpH in the ground state (major pathway). As the result, the quantum yields of the starting compound decomposition under continuous-wave anaerobic photolysis are rather low: 1.6% for NTrpH and 0.02% for KNA. The photolysis of KNA with alpha-crystallin yields the same deoxygenated KNA products as the photolysis of KNA with NTrpH, indicating the similarity of the photolysis mechanisms. Thus, inside the eye lens KNA can sensitize both protein photooxidation and protein covalent cross-linking with the minor self-degradation. This may play an important role in the lens protein modifications during the normal aging and cataract development.


