CetirizineAntihistamine CAS# 83881-51-0 |
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Loratadine
Catalog No.:BCC1262
CAS No.:79794-75-5
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83881-51-0 | SDF | Download SDF |
| PubChem ID | 2678 | Appearance | Powder |
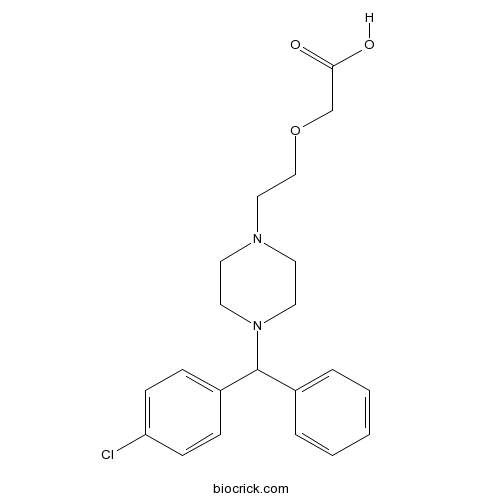
| Formula | C21H25ClN2O3 | M.Wt | 388.89 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO | ||
| Chemical Name | 2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]acetic acid | ||
| SMILES | C1CN(CCN1CCOCC(=O)O)C(C2=CC=CC=C2)C3=CC=C(C=C3)Cl | ||
| Standard InChIKey | ZKLPARSLTMPFCP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cetirizine Dilution Calculator

Cetirizine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5714 mL | 12.8571 mL | 25.7142 mL | 51.4284 mL | 64.2855 mL |
| 5 mM | 0.5143 mL | 2.5714 mL | 5.1428 mL | 10.2857 mL | 12.8571 mL |
| 10 mM | 0.2571 mL | 1.2857 mL | 2.5714 mL | 5.1428 mL | 6.4286 mL |
| 50 mM | 0.0514 mL | 0.2571 mL | 0.5143 mL | 1.0286 mL | 1.2857 mL |
| 100 mM | 0.0257 mL | 0.1286 mL | 0.2571 mL | 0.5143 mL | 0.6429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cetirizine, a second-generation antihistamine, is a major metabolite of hydroxyzine, and a racemic selective H1 receptor inverse agonist used in the treatment of allergies, hay fever, angioedema, and urticaria. Cetirizine crosses the blood-brain barrier only slightly, reducing the sedative side-effect common with older antihistamines. It has also been shown to inhibit eosinophil chemotaxis and LTB4 release. At a dosage of 20 mg, Boone et al. found that it inhibited the expression of VCAM-1 in patients with atopic dermatitis. The levorotary enantiomer of cetirizine, known as levocetirizine, is the more active form. From Wikipedia.
- Obovatol
Catalog No.:BCN8265
CAS No.:83864-78-2
- Angeloylisogomisin O
Catalog No.:BCN4379
CAS No.:83864-70-4
- Angeloylgomisin O
Catalog No.:BCN7361
CAS No.:83864-69-1
- 4-Benzoyl 4'-methyldiphenyl sulfide
Catalog No.:BCC8694
CAS No.:83846-85-9
- 2-Ethylhexyl trans-4-methoxycinnamate
Catalog No.:BCN1333
CAS No.:83834-59-7
- Falecalcitriol
Catalog No.:BCC1570
CAS No.:83805-11-2
- Fmoc-Ser(Bzl)-OH
Catalog No.:BCC3542
CAS No.:83792-48-7
- Fmoc-Arg(Tos)-OH
Catalog No.:BCC3076
CAS No.:83792-47-6
- YM 244769
Catalog No.:BCC6222
CAS No.:837424-39-2
- WH-4-023
Catalog No.:BCC8051
CAS No.:837422-57-8
- Salvinorin A
Catalog No.:BCC5875
CAS No.:83729-01-5
- 3alpha-Acetoxy-20(29)-lupene-23,28-dioic acid
Catalog No.:BCN7508
CAS No.:83725-41-1
- Cetirizine DiHCl
Catalog No.:BCC4517
CAS No.:83881-52-1
- WIKI4
Catalog No.:BCC2455
CAS No.:838818-26-1
- TCS 2312
Catalog No.:BCC7541
CAS No.:838823-31-7
- Azithromycin
Catalog No.:BCC4385
CAS No.:83905-01-5
- Gramodendrine
Catalog No.:BCN2155
CAS No.:83905-67-3
- 12-Acetoxyabietic acid
Catalog No.:BCN4380
CAS No.:83905-81-1
- 13-Hydroxylabda-8(17),14-dien-18-oic acid
Catalog No.:BCN1332
CAS No.:83915-59-7
- Isogomisin O
Catalog No.:BCN4381
CAS No.:83916-76-1
- Mometasone furoate
Catalog No.:BCC4801
CAS No.:83919-23-7
- Flavidin
Catalog No.:BCN6438
CAS No.:83924-98-5
- Flavidinin
Catalog No.:BCN3599
CAS No.:83925-00-2
- 4-Epicommunic acid
Catalog No.:BCN4382
CAS No.:83945-57-7
Combined Effect of Synthetic and Natural Polymers in Preparation of Cetirizine Hydrochloride Oral Disintegrating Tablets: Optimization by Central Composite Design.[Pubmed:28154771]
J Pharm (Cairo). 2017;2017:8305976.
Our aim was to employ experimental design to formulate and optimize Cetirizine hydrochloride oral disintegrating tablets (ODTs) by direct compression technique, using the mutual effect of synthetic croscarmellose sodium (CCS) and natural Hibiscus rosa-sinensis mucilage (HRM) as disintegrants in the formulation. Central composite design (CCD) was applied to optimize the influence of three levels each of CCS (X1) and HRM (X2) concentrations (independent variables) for investigated responses: disintegration time (DT) (Y1), % friability (F) (Y2), and % cumulative drug release (DR) (Y3) (dependent variables). This face-centered second-order model's reliability was verified by the probability and adequate precision values from the analysis of variance, while the significant factor effects influencing the studied responses were identified using multiple linear regression analysis. Perturbation and response surface plots were interpreted to evaluate the responses' sensitivity towards the variables. During optimization, the concentrations of the processed factors were evaluated, and the resulting values were in good agreement with predicted estimates endorsing the validity. Spectral study by Fourier Transform Infrared Spectroscopy (FTIR) and thermograms from Differential Scanning Calorimetry (DSC) demonstrated the drug-excipients compatibility of the optimized formulation. The optimized formulation has concentrations of 9.05 mg and 16.04 mg of CCS and HRM each, respectively, and the model predicted DT of 13.271 sec, F of 0.498, and DR of 99.768%.
Original research paper. Characterization and taste masking evaluation of microparticles with cetirizine dihydrochloride and methacrylate-based copolymer obtained by spray drying.[Pubmed:28231047]
Acta Pharm. 2017 Mar 1;67(1):113-124.
Taste of a pharmaceutical formulation is an important parameter for the effectiveness of pharmacotherapy. Cetirizine dihydrochloride (CET) is a second-generation antihistamine that is commonly administered in allergy treatment. CET is characterized by extremely bitter taste and it is a great challenge to successfully mask its taste; therefore the goal of this work was to formulate and characterize the microparticles obtained by the spray drying method with CET and poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate 1:2:1 copolymer (Eudragit E PO) as a barrier coating. Assessment of taste masking by the electronic tongue has revealed that designed formulations created an effective taste masking barrier. Taste masking effect was also confirmed by the in vivo model and the in vitro release profile of CET. Obtained data have shown that microparticles with a drug/polymer ratio (0.5:1) are promising CET carriers with efficient taste masking potential and might be further used in designing orodispersible dosage forms with CET.
Acute Focal Dystonia After a Single Dose of Oral Cetirizine in a 9-Year-Old Boy.[Pubmed:28169979]
Pediatr Emerg Care. 2019 Feb;35(2):e30-e31.
Common cold is an acute illness affecting pediatric population in particular. The use of antihistamines is a common practice, with Cetirizine being a frequently used drug with a good safety profile. However, adverse events due to the use of antihistamines have been rarely reported, such as drug-induced dystonia with the use of Cetirizine. In our present case, dystonia due to the intake of Cetirizine was observed, which the patient responded well to the use of benzodiazapines, namely, clonazepam. We report this case to highlight the occurrence of this adverse event with the use of Cetirizine.
Toxic effects of the antihistamine cetirizine in mussel Mytilus galloprovincialis.[Pubmed:28273617]
Water Res. 2017 May 1;114:316-326.
Recent studies have become increasingly focused on the assessment of pharmaceuticals occurrence in aquatic ecosystems, however the potential toxicity to non-target organisms is still largely unknown. The antihistamine Cetirizine is a commonly used pharmaceutical, already detected in surface waters of marine aquatic systems worldwide. In the present study Mytilus galloprovincialis mussels were exposed to a range of Cetirizine concentrations (0.3, 3.0, 6.0 and 12.0 mug/L), resembling moderate to highly contaminated areas, over 28 days. The responses of different biochemical markers were evaluated in mussels whole soft tissue, and included energy-related parameters (glycogen content, GLY; protein content, PROT; electron transport system activity, ETS), and oxidative stress markers (superoxide dismutase activity, SOD; catalase activity, CAT; glutathione S-transferases activity, GSTs; lipid peroxidation levels, LPO; reduced (GSH) and oxidized (GSSG) glutathione content). The results obtained demonstrated that with the increase of exposure concentrations mussels tended to increase their energy reserves and maintain their metabolic potential, which was significantly higher only at the highest concentration. Our findings clearly revealed that Cetirizine inhibited the activity of GSTs and although induced the activity of antioxidant enzymes (SOD and CAT) mussels were not able to prevent cellular damages observed through the increase of LPO associated to the increase of exposure concentrations. Thus, this study confirmed that Cetirizine induces toxic effects in Mytilus galloprovincialis, which, considering their trophic relevance, wide use as bioindicator and wide spatial distribution of this species, can result in ecological and economic negative impacts at a large scale.


