ObovatolCAS# 83864-78-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 83864-78-2 | SDF | Download SDF |
| PubChem ID | 100771 | Appearance | Oil |
| Formula | C18H18O3 | M.Wt | 282.3 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
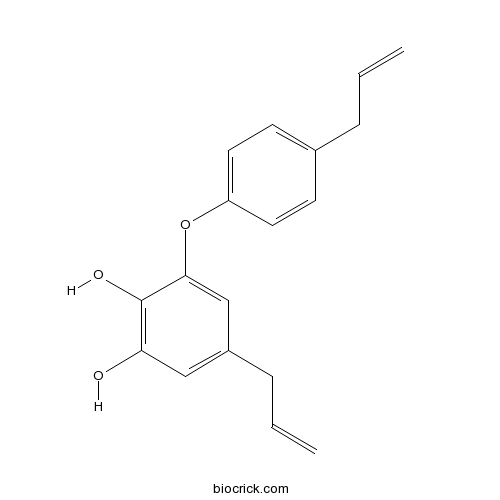
| Chemical Name | 5-prop-2-enyl-3-(4-prop-2-enylphenoxy)benzene-1,2-diol | ||
| SMILES | C=CCC1=CC=C(C=C1)OC2=CC(=CC(=C2O)O)CC=C | ||
| Standard InChIKey | OPGPFZQBCIAFLI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H18O3/c1-3-5-13-7-9-15(10-8-13)21-17-12-14(6-4-2)11-16(19)18(17)20/h3-4,7-12,19-20H,1-2,5-6H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Obovatol has antioxidant, neuroprotective, antiinflammatory, antithrombotic and antitumour effects. 2. Obovatol is a potent NF-κB inhibitors for Alzheimer's disease treatment. 3. Obovatol shows inhibitory effect on the Salmonella type III secretion system, it could be useful for the prevention and supplementary treatment of bacterial infections. 4. Obovatol inhibits the growth and aggressiveness of tongue squamous cell carcinoma through regulation of the EGF-mediated JAK-STAT signaling pathway. 5. Obovatol induces apoptosis via CHOP activation in A549 and H460 NSCLCs. 6. Obovatol inhibits receptor activator of nuclear factor kappa B (NF-κB) ligand (RANKL)-induced osteoclast differentiation in vitro and inflammatory bone loss in vivo, it may be a useful therapeutic agent for the treatment of pathological bone disorders characterized by excessive osteoclastic bone resorption. 7. Obovatol shows neuroprotective effects against glutamate-induced apoptotic stimuli in HT22 cells. 8. Obovatol has a potent antithrombotic effect, which may be due to antiplatelet activity. 9. Obovatol inhibits vascular smooth muscle cell proliferation and intimal hyperplasia by inducing p21Cip1, it may have potential as an anti-proliferative agent for the treatment of restenosis and atherosclerosis. |
| Targets | Bcl-2/Bax | Caspase | JAK | STAT | EGFR | NF-kB | PARP | p38MAPK | COX | LOX | Calcium Channel | p21 |
| Animal Research | Obovatol from Magnolia obovata inhibits vascular smooth muscle cell proliferation and intimal hyperplasia by inducing p21Cip1.[Pubmed: 20022323]Atherosclerosis. 2010 Jun;210(2):372-80.Obovatol is isolated from Magnolia obovata leaves and this active component has various pharmacological properties such as anti-oxidant, anti-platelet, anti-fungal and anti-inflammatory activities. In the present study, we investigated the inhibitory effects of Obovatol on in vitro vascular smooth muscle cell (VSMC) proliferation and in vivo neointimal formation in a rat carotid artery injury model. |

Obovatol Dilution Calculator

Obovatol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5423 mL | 17.7117 mL | 35.4233 mL | 70.8466 mL | 88.5583 mL |
| 5 mM | 0.7085 mL | 3.5423 mL | 7.0847 mL | 14.1693 mL | 17.7117 mL |
| 10 mM | 0.3542 mL | 1.7712 mL | 3.5423 mL | 7.0847 mL | 8.8558 mL |
| 50 mM | 0.0708 mL | 0.3542 mL | 0.7085 mL | 1.4169 mL | 1.7712 mL |
| 100 mM | 0.0354 mL | 0.1771 mL | 0.3542 mL | 0.7085 mL | 0.8856 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Angeloylisogomisin O
Catalog No.:BCN4379
CAS No.:83864-70-4
- Angeloylgomisin O
Catalog No.:BCN7361
CAS No.:83864-69-1
- 4-Benzoyl 4'-methyldiphenyl sulfide
Catalog No.:BCC8694
CAS No.:83846-85-9
- 2-Ethylhexyl trans-4-methoxycinnamate
Catalog No.:BCN1333
CAS No.:83834-59-7
- Falecalcitriol
Catalog No.:BCC1570
CAS No.:83805-11-2
- Fmoc-Ser(Bzl)-OH
Catalog No.:BCC3542
CAS No.:83792-48-7
- Fmoc-Arg(Tos)-OH
Catalog No.:BCC3076
CAS No.:83792-47-6
- YM 244769
Catalog No.:BCC6222
CAS No.:837424-39-2
- WH-4-023
Catalog No.:BCC8051
CAS No.:837422-57-8
- Salvinorin A
Catalog No.:BCC5875
CAS No.:83729-01-5
- 3alpha-Acetoxy-20(29)-lupene-23,28-dioic acid
Catalog No.:BCN7508
CAS No.:83725-41-1
- Pomolic acid 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN1334
CAS No.:83725-24-0
- Cetirizine
Catalog No.:BCC1469
CAS No.:83881-51-0
- Cetirizine DiHCl
Catalog No.:BCC4517
CAS No.:83881-52-1
- WIKI4
Catalog No.:BCC2455
CAS No.:838818-26-1
- TCS 2312
Catalog No.:BCC7541
CAS No.:838823-31-7
- Azithromycin
Catalog No.:BCC4385
CAS No.:83905-01-5
- Gramodendrine
Catalog No.:BCN2155
CAS No.:83905-67-3
- 12-Acetoxyabietic acid
Catalog No.:BCN4380
CAS No.:83905-81-1
- 13-Hydroxylabda-8(17),14-dien-18-oic acid
Catalog No.:BCN1332
CAS No.:83915-59-7
- Isogomisin O
Catalog No.:BCN4381
CAS No.:83916-76-1
- Mometasone furoate
Catalog No.:BCC4801
CAS No.:83919-23-7
- Flavidin
Catalog No.:BCN6438
CAS No.:83924-98-5
- Flavidinin
Catalog No.:BCN3599
CAS No.:83925-00-2
Obovatol Induces Apoptosis in Non-small Cell Lung Cancer Cells via C/EBP Homologous Protein Activation.[Pubmed:27489231]
Phytother Res. 2016 Nov;30(11):1841-1847.
Although Obovatol, a phenolic compound from the bark of Magnolia obovata, was known to have antioxidant, neuroprotective, antiinflammatory, antithrombotic and antitumour effects, its underlying antitumour mechanism is poorly understood so far. Thus, in the present study, the antitumour molecular mechanism of Obovatol was investigated in non-small cell lung cancer cells (NSCLCs). Obovatol exerted cytotoxicity in A549 and H460 NSCLCs, but not in BEAS-2B cells. Also, Obovatol increased sub-G1 accumulation and early and late apoptotic portion in A549 and H460 NSCLCs. Consistently, Obovatol cleaved PARP, activated caspase 9/3 and Bax and attenuated the expression of cyclin D1 in A549 and H460 NSCLCs. Interestingly, Obovatol upregulated the expression of endoplasmic reticulum stress proteins such as C/EBP homologous protein (CHOP), IRE1alpha, ATF4 and p-elF2 in A549 and H460 NSCLCs. Conversely, depletion of CHOP blocked the apoptotic activity of Obovatol to increase sub-G1 accumulation in A549 and H460 NSCLCs. Overall, our findings support scientific evidences that Obovatol induces apoptosis via CHOP activation in A549 and H460 NSCLCs. Copyright (c) 2016 John Wiley & Sons, Ltd.
RANKL-induced osteoclastogenesis is suppressed by 4-O-methylhonokiol in bone marrow-derived macrophages.[Pubmed:28736799]
Arch Pharm Res. 2017 Aug;40(8):933-942.
Magnolol, honokiol, and Obovatol are well known bioactive constituents of the bark of Magnolia officinalis and have been reported to have beneficial effects in various diseases. We recently isolated a novel active compound, 4-O-methylhonokiol (4-O-MH) from the ethanol extract of M. officinalis, which was previously reported to have pharmacological effects including anti-inflammatory, anti-oxidative, and anti-aging activities. Here, we examined the pharmacological properties of 4-O-MH on osteoblast (bone-forming cells) and osteoclast (bone-resorbing cells) differentiation, and its underlying signaling pathways in primary cultured pre-osteoblasts and bone marrow macrophages. Our results showed that 4-O-MH did not affect cell viability in pre-osteoblasts and did not influence osteoblast differentiation and mineralized nodule formation, as assessed by alkaline phosphatase activity and Alizarin red staining. However, 4-O-MH significantly inhibited TRAP-positive multinuclear osteoclasts and F-actin ring formation during Receptor activator of NF-kappaB ligand (RANKL)-mediated osteoclastogenesis without cytotoxicity. In addition, 4-O-MH suppressed RANKL-induced critical factors (c-Fos, NF-ATc1, TRAP, and ITB3) for osteoclast differentiation and function. Furthermore, RANKL-mediated signaling, including ERK1/2, AKT, and NF-kB pathways was attenuated by 4-O-MH. Taken together, 4-O-MH has an inhibitory role in RANKL-mediated osteoclastogenesis but not osteoblast differentiation, and our findings also suggest that 4-O-MH is a potential therapeutic agent for bone-destructive diseases such as osteoporosis, alveolar bone resorption, and osteoarthritis.
Effect of Chemical Profiling Change of Processed Magnolia officinalis on the Pharmacokinetic Profiling of Honokiol and Magnolol in Rats.[Pubmed:27107095]
J Chromatogr Sci. 2016 Aug;54(7):1201-12.
The stem of Magnoliae officinalis (MO) cortex is always preliminarily processed before being applied in traditional Chinese medicine. The definite bioavailability of honokiol (HO) and magnolol (MA) in processed MO (PMO) and the effect of chemical profiling change on the pharmacokinetics of HO and MA are always a greater challenge compared with those of MO. Compared with that of MO, the pharmacokinetic profiling of HO and MA in the PMO was significantly changed and the mean Tmax of HO and MA was increased by 31 and 50% (P < 0.05), respectively; the mean AUC0-t and Cmax of HO were increased by 36 and 24% (P < 0.05), respectively. Subsequently, the chemical profiling of MO and PMO was investigated by a simple and rapid LC-Q/TOF-MS coupled with multivariate analysis method. Principal component analysis and hierarchical cluster analysis of the chromatographic data demonstrated that the chemical profiling of PMO was significantly different from that of MO. Eight marker components including six alkaloids (magnocurarine, magnoflorine, roemerine and three unidentified peaks) and two lignans (Obovatol and MA) were screened out by partial least-squares discriminant analysis. The results indicated that the changes of eight marker components of PMO may have an effect on the pharmacokinetic profiles of HO and MA.
Sesquiterpene-neolignans from Manglietia hookeri.[Pubmed:26609630]
Nat Prod Res. 2016 Jul;30(13):1477-83.
The comet assay-guided fractionation of the twigs of Manglietia hookeri resulted in the isolation of three sesquiterpene-neolignans, including a new one 5-allyl-2-(4-allyl-phenoxy)-3-[7-(1-hydroxy-1-methyl-ethyl)-1, 4a-dimethyl-decahydro-naphthalen-1-yloxy]-phenol (1), and eudesObovatol A (2) and eudesObovatol B (3), together with three lignans, Obovatol (4), honokiol (5) and magnolol (6). Their structures were elucidated on the basis of spectral analysis and by comparison with related literature data. Compounds 1, 4-6 showed a protective effect on UV inductive DNA damage in mice lymphocyte cells, while compound 1 indicated the smallest Olive Tail Moment 7.34 +/- 2.09 at 6 x 10(-6) muM.
Phytochemicals as inhibitors of NF-kappaB for treatment of Alzheimer's disease.[Pubmed:29179999]
Pharmacol Res. 2018 Mar;129:262-273.
Alzheimer's disease (AD) is the most prevalent form of dementia. The exact pathophysiology of this disease remains incompletely understood and safe and effective therapies are required. AD is highly correlated with neuroinflammation and oxidative stress in brain causing neuronal loss. Nuclear factor of activated B-cells (NF-kappaB) is involved in physiological inflammatory processes and thus representing a promising target for inflammation-based AD therapy. Phytochemicals are able to interfere with the NF-kappaB pathway. They inhibit the phosphorylation or the ubiquitination of signaling molecules, and thus, inhibit the degradation of IkappaB. The translocation of NF-kappaB to the nucleus and subsequent transcription of pro-inflammatory cytokines are inhibited by the actions of phytochemicals. Additionally, natural compounds preventing the interaction of NF-kappaB can block NF-kappaB's transcriptional activity by inhibiting its binding to target DNA. Many polyphenols including curcumin, resveratrol, pterostilbene, punicalagin, macranthoin G, salidroside, 4-O-methylhonokiol, lycopene, genistein, Obovatol and gallic acid were reported as potent NF-kappaB inhibitors for AD treatment. Several alkaloids such as galantamine, glaucocalyxin B, tetrandrine, berberine, oridonin, anatabine have been shown anti-inflammatory effects in AD models in vitro as well as in vivo. Besides, vitamins, tanshinone IIA, artemisinin, dihydroasparagusic acid, geniposide, xanthoceraside, l-theranine, 1,8-cineole and paeoniflorin were described as promising NF-kappaB inhibitors. In conclusion, natural products from plants represent interesting candidates for AD treatment. They may qualify as promising compounds for the development of derivatives providing enhanced pharmacological features.
Inhibitory effect of obovatol from Magnolia obovata on the Salmonella type III secretion system.[Pubmed:28874849]
J Antibiot (Tokyo). 2017 Nov;70(11):1065-1069.
In many pathogenic Gram-negative bacteria, such as Salmonella, Escherichia coli, Yersinia and Chlamydia spp., which cause diseases in humans, the type III secretion system (TTSS) is an important virulence factor that translocates effector proteins into the cytosol of host cells. Thus, the TTSS is a good target for antibacterial agents. Here we used a hemolysis assay to search for TTSS inhibitors and found that a compound from Magnolia obovata called Obovatol blocks the TTSS of Salmonella. Obovatol showed potent inhibitory activity (IC50=19.8 muM) against the TTSS-related hemolysis of Salmonella, which was not due to a reduction of bacterial growth. Instead, the compound inhibited bacterial motility, TTSS-related mRNA expression and effector protein secretion. These data demonstrate the inhibitory effect of Obovatol on the Salmonella TTSS and suggest that it could be useful for the prevention and supplementary treatment of bacterial infections.
Induction of apoptosis by obovatol as a novel therapeutic strategy for acute myeloid leukemia.[Pubmed:25319672]
Int J Mol Med. 2014 Dec;34(6):1675-80.
Obovatol, a compound isolated from the bark cortex of Magnolia officinalis (cortex Magnoliae officinalis; M. officinalis), has been studied for use in the treatment of solid cancers. However, the mechanisms of action and the effects of Obovatol against acute myeloid leukemia (AML) remain unclear and require further investigation. Therefore, this study was conducted using a human AML cell line (MM6). Obovatol increased pro-apoptotic (Bax) and decreased anti-apoptotic (Bcl-2) protein expression, resulting in caspase-3 and caspase-9 activation measured by caspase-Glo 3/7 assay. Furthermore, Obovatol activated the mitogen-activated protein kinase (MAPK) signaling pathway [c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38] and inhibited the activation of the nuclear factor-kappaB (NF-kappaB) signaling pathway analyzed by western blot analysis. Taken together, these findings provide evidence that Obovatol inhibits cell proliferation in AML and induces apoptosis through the activation of the MAPK pathway in addition to the intrinsic apoptotic pathway. In addition, Obovatol suppressed the expression of mixed-lineage leukemia (MLL) target genes by inhibiting the activation of the NF-kappaB pathway. Therefore, these results suggest that Obovatol may have potential for use in the treatment of leukemia.


