Stephania tetrandra
Stephania tetrandra
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Stephania tetrandra
- Cat.No. Product Name CAS Number COA
-
BCN2624
Demethyl tetrandrine33889-68-8
Instructions
-
BCN5956
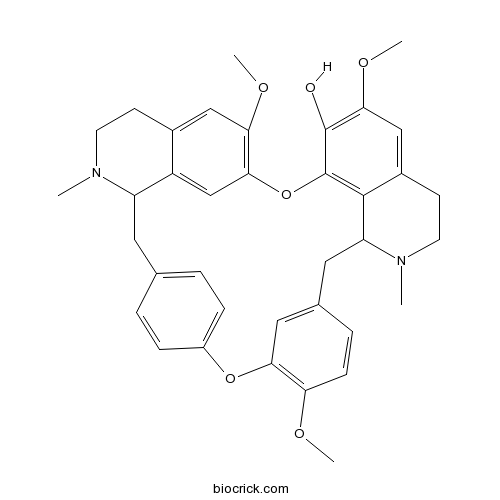
Fangchinoline436-77-1
Instructions

Screening and investigation of triplex DNA binders from Stephania tetrandra S. Moore by a combination of peak area-fading ultra high-performance liquid chromatography with orbitrap mass spectrometry and optical spectroscopies.[Pubmed: 29763521]
The identification and screening of triplex DNA binders are important because these compounds, in many cases, are potential anticancer agents as well as promising drug candidates. Therefore, the ability to screen for these compounds in a high-throughput mode could dramatically improve the drug screening process. A method involving a combination of 96-well plate format and peak area-fading ultra high-performance liquid chromatography coupled with Orbitrap mass spectrometry was employed for screening bioactive compounds binding to the triplex DNA from the extracts of Stephania tetrandra S. Moore. Two compounds were screened out and identified as fangchinoline and tetrandrine based on the comparison of retention time and tandem mass spectrometry data with those of standards. The binding mechanisms of fangchinoline and tetrandrine at the molecular level were explored using tandem mass spectrometry, fluorescence spectroscopy, ultraviolet-visible spectroscopy, and circular dichroism. Collision-induced dissociation experiments showed that the complexes with fangchinoline and tetrandrine were dissociated by ligand elimination. According to these measurements, an intercalating binding is the most appropriate binding mode of these two alkaloids to the triplex DNA. The current work provides not only deep insight into alkaloid-triplex DNA complexes but also useful guidelines for the design of efficient anticancer agents.
[Pharmacological Mechanisms of Boiogito and Bofutsushosan in Diabetes and Obesity Models].[Pubmed: 29503432]
The antihyperglycemic activities of extracts of boiogito (BOT) and bofutsushosan (BTS) were investigated in streptozotocin-induced (STZ)-diabetic mice. BOT extract containing Stephania tetrandra S. MOORE root (stephania), has more potent antihyperglycemic activity than BOT extract containing sinomenium stem (sinomenium). Extracts of stephania and astragalus root (astragalus) exert combined effects in the antihyperglycemic and insulinotropic activities of BOT extract. Fangchinoline, but not tetrandrine, in stephania plays a role in its activity. Formononetin in astragalus potentiates the actions of fangchinoline. Tetrandrine has antiangiogenic effects on choroidal vessels in STZ-diabetic rats, which are associated with the inhibition of tumor necrosis factor (TNF)-α-induced nuclear factor (NF)-κB activation. BTS extract has shown antihyperglycemic and insulinotropic activities whereas gardenia fruit (gardenia) extract in BTS has antihyperglycemic, but not insulinotropic, activity in the diabetic mice. Gardenia extract decreased the HOMA-IR level and increased insulin-stimulated 2-deoxyglucose (2-DG) uptake to skeletal muscle. The effects of gardenia extract on 2-DG uptake were associated with the upregulation of glucose transporter type 4 and Akt phosphorylation. Gardenia extract was also shown to have antihyperglycemic and insulinotropic actions in high-fat diet (HFD)-fed and STZ-diabetic mice. In addition, gardenia extract decreased the production of TNF-α and leptin, and increased the production of adiponectin in the visceral adipose tissues. In the early administration period, BTS extract increased mRNA expression levels of leptin, adiponectin, and UCP1 in brown adipose tissues in HFD-fed obese mice. With a longer duration of administration, BTS extract improved insulin resistance and subsequently reduced serum leptin and triglyceride levels in parallel with visceral adipose tissue volume and size.
The plant alkaloid tetrandrine inhibits metastasis via autophagy-dependent Wnt/β-catenin and metastatic tumor antigen 1 signaling in human liver cancer cells.[Pubmed: 29334999]
Tetrandrine is a bisbenzylisoquinoline alkaloid isolated from the Chinese medicinal herb Stephania tetrandra S. Moore. We previously demonstrated that tetrandrine exhibits potent antitumor effects in many types of cancer cells. In this study, we investigated the effects of tetrandrine on human hepatocellular carcinoma (HCC) metastasis.
Autophagy induction enhances tetrandrine-induced apoptosis via the AMPK/mTOR pathway in human bladder cancer cells.[Pubmed: 29048631]
Tetrandrine, a bisbenzylisoquinoline alkaloid isolated from the roots of Stephania tetrandra is a traditional Chinese medicine and exerts anticancer capacity in various types of cancers. Previous studies have shown that tetrandrine induces apoptosis in bladder cancer cells via activation of the caspase cascade. However, the underlying mechanism has not yet been reported. Autophagy is a cellular process involved in the degradation of broken proteins and aging organelles to maintain homeostasis. Recent studies indicate that autophagy is implicated in cancer therapy. Thus, we focused on the correlation between autophagy and apoptosis upon tetrandrine treatment in human bladder cancer cells. Firstly, our results observed a marked increase in autophagic double-membrane vacuoles and fluorescent puncta of red fluorescence protein-green fluorescence protein-LC3 (GRP-RFP-LC3) upon tetrandrine treatment, as evidenced by transmission electron microscopy and confocal fluorescence microscopy. Secondly, the expression of LC3-II was increased in tetrandrine-treated T24 and 5637 cells in a time- and concentration-dependent manner. Subsequently, downregulation of p62 and LC3 turnover assay further confirmed that tetrandrine induced autophagic flux in bladder cancer T24 and 5637 cells. Thirdly, the protein levels of phosphorylated-AMP-activated protein kinase (AMPK) and phosphorylated-acetyl-coenzyme A carboxylase (ACC) were upregulated in the tetrandrine-treated cells, while the mammalian target of rapamycin (mTOR)-related proteins were downregulated. Moreover, AICAR, a common AMPK activator, further increased the expression the LC3-II, while AMPK inhibitor compound C partially reversed the LC3-II protein levels in bladder cancer T24 cells. Finally, AICAR significantly reinforced the growth inhibition and apoptosis induction of tetrandrine in T24 and 5637 cells, while compound C had an opposite effect, suggesting that AMPK-mediated autophagy enhanced the cytotoxic and pro-apoptosis effect of tetrandrine in human bladder cancer cells. Taken together, the present study showed that tetrandrine induced autophagy in human bladder cancer cells by regulating the AMPK/mTOR signaling pathway, which contributed to the apoptosis induction by tetrandrine, indicating that tetrandrine may be a potential anticancer candidate for the treatment of bladder cancer, and autophagy may be a possible mechanism for cancer therapy.
Fangchinoline Induces Apoptosis, Autophagy and Energetic Impairment in Bladder Cancer.[Pubmed: 28968601]
Tetrandrine and Fangchinoline (Fcn) are two natural products that are found in Stephania tetrandra. Tetrandrine is a known anti-bladder cancer compound, but the effects of Fcn on bladder cancer have been previously unclear. In the present study, we focused on the anti-tumor effects of Fcn on bladder cancer.
Inactivation of the orphan nuclear receptor NR4A1 contributes to apoptosis induction by fangchinoline in pancreatic cancer cells.[Pubmed: 28754437]
Previous studies have demonstrated that the orphan nuclear receptor NR4A1 is overexpressed in human pancreatic cancer and antagonizing this receptor promotes apoptosis and inhibits pancreatic cancer cells and tumor growth. In the present study, we identified fangchinoline, a bisbenzyltetrahydroisoquinoline alkaloid from Stephania tetrandra, as a new inactivator of nuclear NR4A1 and demonstrated that fangchinoline inhibits cell proliferation and induces apoptosis, in part, via the NR4A1-dependent pro-apoptotic pathways in human pancreatic cancer cells. It decreased expression of the antiapoptotic protein survivin by inhibiting Sp1-mediated transcription and induced oxidative stress-mediated endoplasmic reticulum (ER) stress in pancreatic cancer cells. These results suggest that inhibition of NR4A1-mediated transcriptional activity was involved in the anticancer effects of fangchinoline, and fangchinoline represents a novel class of mechanism-based anticancer agents targeting NR4A1 that is overexpressed in pancreatic cancer.


