Styrax tonkinensis
Styrax tonkinensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Styrax tonkinensis
- Cat.No. Product Name CAS Number COA
-
BCN2605
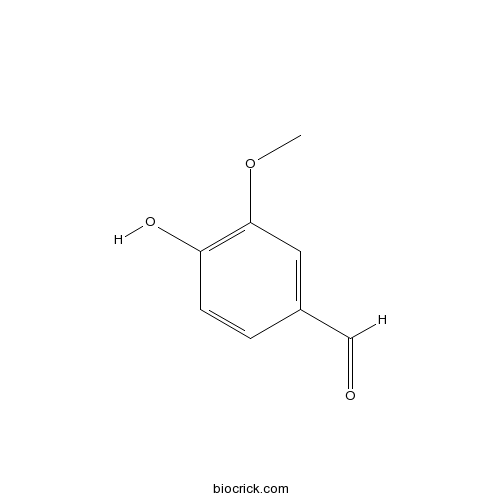
Vanillin121-33-5
Instructions
Two new triterpenoids from the resin of Styrax tonkinensis.[Pubmed: 25908248]
Two new triterpenoids, 3β,6β-dihydroxy-12-oxo-13Hα-olean-28,19β-olide (1) and 3-oxo-olean-11,13(18)-dien-28,19β-olide (2) were isolated from the resin of Styrax tonkinensis (Pier.) Craib. The structures of both triterpenoids were determined by physicochemical and spectroscopic methods. Compound 1 is the second triterpene found with cis-fused C/D ring from the resin, which is rarely observed in oleanane-type triterpenes.
Seedling growth and metal accumulation of selected woody species in copper and lead/zinc mine tailings.[Pubmed: 21517000]
A greenhouse pot experiment was conducted to evaluate the potential of selected woody plants for revegetation in copper (Cu) and lead/zinc (Pb/Zn) mine tailing areas. Five woody species (Amorpha fruticosa Linn, Vitex trifolia Linn. var. simplicifolia Cham, Glochidion puberum (Linn.) Hutch, Broussonetia papyrifera, and Styrax tonkinensis) and one herbaceous species (Sesbania cannabina Pers) were planted in Cu and Pb/Zn tailings to assess their growth, root morphology, nutrition uptake, metal accumulation, and translocation in plants. Amorpha fruticosa maintained normal growth, while the other species demonstrated stress related growth and root development. Sesbania cannabina showed the highest biomass among the plants, although it decreased by 30% in Cu tailings and 40% in Pb/Zn tailings. Calculated tolerance index (TI) values suggested that A. fruticosa, an N-fixing shrub, was the most tolerant species to both tailings (TI values 0.92-1.01), while S. cannabina had a moderate TI of 0.65-0.81 and B. papyrifera was the most sensitive species, especially to Pb/Zn tailings (TI values 0.15-0.19). Despite the high concentrations of heavy metals in the mine tailings and plants roots, only a small transfer of these elements to the aboveground parts of the woody plants was evident from the low translocation factor (TF) values. Among the woody plants, V. trifolia var. simplicifolia had the highest TF values for Zn (1.32), Cu (0.78), and Pb/Zn (0.78). The results suggested that A. fruticosa and S. cannabina, which have the highest tolerance and biomass production, respectively, demonstrated the potential for tailings revegetation in southern China.
Two new aromatic compounds from the resin of Styrax tonkinensis (Pier.) Craib.[Pubmed: 16753795]
Two new aromatic compounds, trans-(tetrahydro-2-(4-hydroxy-3-methoxyphenyl)-5-oxofuran-3-yl)methyl benzoate (1), 3-(4-hydroxy-3-methoxyphenyl)-2-oxopropyl benzoate (2) and one new natural product, 4-((E)-3-ethoxyprop-1-enyl)-2-methoxyphenol (3) together with five known aromatic compounds have been isolated from the resin of Styrax tonkinensis (Pier.) Craib. Their structures were determined by physical and spectroscopic methods.
Triterpenoids from the resin of Styrax tonkinensis and their antiproliferative and differentiation effects in human leukemia HL-60 cells.[Pubmed: 16724846]
Four new triterpenoids, 6beta-hydroxy-3-oxo-11alpha,12alpha-epoxyolean-28,13beta-olide (1), 3beta,6beta-dihydroxy-11alpha,12alpha-epoxyolean-28,13beta-olide (2), 3beta,6beta-dihydroxy-11-oxo-olean-12-en-28-oic acid (3), and 3beta-hydroxy-12-oxo-13Halpha-olean-28,19beta-olide (4), and five known triterpenes, 19alpha-hydroxy-3-oxo-olean-12-en-28-oic acid (5), 6beta-hydroxy-3-oxo-olean-12-en-28-oic acid (6), sumaresinolic acid (7), siaresinolic acid (8), and oleanolic acid (9), were isolated from the resin of Styrax tonkinensis. The structures of these triterpenoids were determined by physicochemical and spectroscopic methods. The configuration of compound 4 was confirmed by X-ray crystallographic analysis. All these triterpenoids inhibited HL-60 cell growth with IG(50) values ranging from 8.9 to 99.4 microM. Oleanolic acid (9) was the most effective antiproliferative agent, with an IG(50) value of 8.9 microM. While 3beta,6beta-dihydroxy-11-oxo-olean-12-en-28-oic acid (3) exhibited the least effective growth inhibition among these triterpenoids, it induced HL-60 cells to undergo differentiation as measured by an NBT reduction assay.
Anti-Aspergillus activities of plant essential oils and their combination effects with ketoconazole or amphotericin B.[Pubmed: 12785735]
The essential oils from Cedrus atlantica, Styrax tonkinensis, Juniperus communis, Lavandula angustifolia, Melaleuca alternifolia, Pelargonium graveolens, Pogesternon patchouli and Rosmarinus officinalis were analyzed by GC-MS. Antifungal activities of the oils were investigated by disk diffusion assay and the broth dilution method against Aspergillus niger and A. flavus. The effects of geraniol and the essential oil fraction from P. graveolens on the antifungal activity of amphotericin B and ketoconazole were examined using a checkerboard microtiter assay against both Aspergillus fungi. Most of the tested essential oils, with the exception of C. atlantica, J. communis, and P. patchouli, significantly inhibited growth of A. niger and to a lesser extent that of A. flavus, with MICs (minimal inhibitory concentrations) in the range 0.78-12.5 mg/mL. The essential oil fraction of P. graveolens and its main components, geraniol and citronellol, exhibited additive effects with amphotericin B and with ketoconazole against both Aspergillus species, resulting in fractional inhibitory concentration (FIC) indices ranging from 0.52 to 1.00.


