Vitex negundo var. cannabifolia
Vitex negundo var. cannabifolia
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Vitex negundo var. cannabifolia
- Cat.No. Product Name CAS Number COA
-
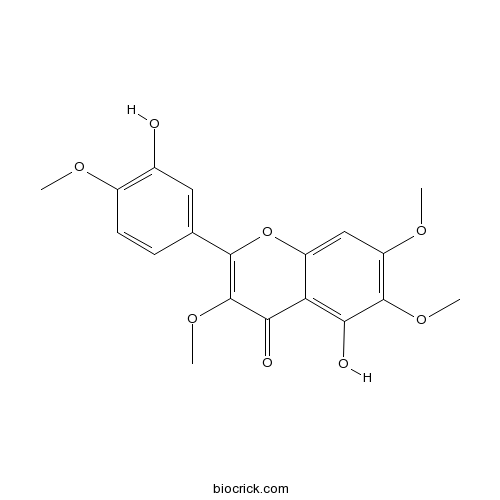
BCN5020
Vitexicarpin479-91-4
Instructions
-
BCN5658
Apigenin520-36-5
Instructions

[Chemical constituents from the fruits of Vitex negundo var. cannabifolia and their biological activities in vitro].[Pubmed: 28933089]
Chemical constituents from the fruits of Vitex negundo var. cannabifolia and their nitric oxide (NO) inhibitory and cytotoxic activities were investigated. The compounds were isolated and purified by various column chromatography, and their structures were identified by physiochemical properties and spectroscopic data. Thirteen lignans and six phenolic compounds were isolated from the CH2Cl2 extract of the fruits of V. negundo var. cannabifolia, respectively. Their structures were elucidated as 6-hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-7-methoxy-3,4-dihydro-2-naphthaldehyde (1), vitedoin A (2), vitexdoin F (3), detetrahydroconidendrin (4), vitexdoin E (5), 4-oxosesamin (6), L-sesamin (7), (+)-beechenol (8), ligballinol (9), 2-(4-hydroxyphenyl)-6-(3-methoxy-4-hydroxyphenyl)-3,7-dioxabicyclo[3.3.0]octane (10), (-)-pinoresinol (11), balanophonin (12), thero-guaiacylglycerol-β-coniferyl aldehyde ether (13), trans-p-coumaryl aldehyde (14), coniferyl aldehyde (15), 5,7-dihydroxychromone (16), trans-3,5-dimethoxy-4-hydroxy-cinnamic aldehyde (17), frambinone (18), and alternariol 4-methyl ether (19). Compounds 8-10,14,18,19 were firstly isolated from Verbenaceae family, compound 13 was obtained from Vitex species, and 6,7,12,15-17 from V. negundo var. cannabifolia for the first time, respectively. The isolated compounds were evaluated for their anti-inflammatory and cytotoxic effects in vitro. Eight compounds (3,5,7,10,11,14,15,17) showed inhibition against NO production in LPS-stimulated RAW 267.4 cells (IC₅₀ in the range of 7.8-81.1 μmol•L⁻¹) and four compounds (1-4) showed cytotoxicity on HepG-2 cells (IC₅₀ in the range of 5.2-24.2 μmol•L⁻¹).
Furofuran lignan glucosides from the leaves of Vitex negundo var. cannabifolia.[Pubmed: 27830592]
None
Chemical constituents and their bioactivities from the fruits of Vitex negundo var. cannabifolia.[Pubmed: 27093612]
None
Anti-inflammatory ursane- and oleanane-type triterpenoids from Vitex negundo var. cannabifolia.[Pubmed: 25245917]
Six new polyoxygenated triterpenoids, cannabifolins A-F (1-6), and eight known triterpenoids, 7-14, were isolated from the leaves of Vitex negundo var. cannabifolia. The absolute configuration of cannabifolin A (1) was determined by single-crystal X-ray crystallographic analysis. Compounds 1 and 2 represent a class of rare natural pentacyclic triterpenoids bearing cis-fused C/D rings and are the first examples of 12,19-epoxy ursane- and oleanane-type triterpenoids. Compounds 3, 7, 8, and 14 exhibited inhibition of nitric oxide production in lipopolysaccharide-induced RAW 264.7 macrophages with IC50 values in the range 24.9-40.5 μM.
Antioxidant lignans from the seeds of Vitex negundo var. cannabifolia.[Pubmed: 24965780]
A new phenyldihydronaphthalene-type lignan, vitexdoin F (1), along with 22 known lignan derivatives (2-23) was isolated from the seeds of Vitex negundo var. cannabifolia. Their structures were established by comprehensive 1D and 2D NMR spectroscopic analyses. The antioxidant activities of these lignans were evaluated through 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging assays, and 16 of these isolates exhibited obvious radical-scavenging effect on the stable-free radical, DPPH.
[Isolation and identification of endophytic fungi from Vitex negundo var. cannabifolia].[Pubmed: 22016965]
The research aimed to investigate the entophytic fungal community of Vitex negundo var. cannabifolia, including the biodiversity from different organs and the correlations with habitations.
Antiseptic activity and phenolic constituents of the aerial parts of Vitex negundo var. cannabifolia.[Pubmed: 21088661]
Four phenolics, salviaplebeiaside (1), γ-tocopherol (2), chrysosplenol-D (4), and isovitexin (5), along with α-tocoquinone (3) and β-sitosterol (6) were isolated from the aerial parts of Vitex negundo var. cannabifolia. The isolation was performed using bio-assay tracking experiments. The structures of compounds 1-5 were established by spectroscopic means. The antibacterial activities of the compounds were assessed against Escherichia coli, Bacillus subtilis, Micrococcus tetragenus and Pseudomonas fluorescens. Chrysosplenol-D (4) exhibited activities against all the four spoilage microorganisms.
[Interspecific association between understory species in a southern highland plantation].[Pubmed: 16471332]
Based upon 2 x 2 contingency table, chi2 test and association coefficient were used to determine the interspecific association between understory species in a southern highland plantation, and to analyze the restoration degree and the stability of southern highland vegetations originated from plantation. The Qianyanzhou in Taihe County of Jiangxi Province, a typical sample of southern highland plantation, was chosen to make the study. The results showed that both in shrub layer and in herb layer, species pair with chi2 reaching significant level (P <0.05) was few in number. In shrub layer, 12 species pairs' association was highly significant (P < 0.01), 19 pairs' was significant (P < 0.05), and other 200 pairs' was nonsignificant, while in herb layer, 11 pairs' was highly significant, 11 pairs' was significant and other 83 pairs' was nonsignificant. According to interspecific association and correlation, shrub layer was divided into two species groups: Group I . Adinandra bockiana, Syzygiumn grijsii, Vaccinium bracteatunm, Ilex aculeolata, Smilax ferox, Eurya muricata and Group II . Lespedeza davidii, Serissa serissoides, Vitex negundo var. cannabifolia. Many species in Group I had a significantly negative association with the species in Group II, and dominant species always played a key role in the relationships among species. The three dominant species in herb layer, Wooduardia japonica, Dryopteris atrata and Adiantun flabellulaturn, had a highly significant positive correlation between each other, and moreover, had a significant or highly significant positive association with many other herbaceous species. Similarily, dominant species in shrub layer played a key role on the interspecific association in the two species groups. The ratios of positive and negative association indicating the species compositions of the two layers were fluctuating, which was 125/106 in shrub layer and 42/63 in herb layer. Several shortcomings of interspecific association method were pointed out, with some proposals put forward.


