VitexicarpinCAS# 479-91-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 479-91-4 | SDF | Download SDF |
| PubChem ID | 5315263 | Appearance | Yellow powder |
| Formula | C19H18O8 | M.Wt | 374.34 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 3',5-Dihydroxy 3,4',6,7-tetramethoxyflavone; Quercetagetin 3,4',6,7-tetramethyl ether; 3,4',6,7-Tetra-O-methylquercetagetin; Vitexicarpin | ||
| Solubility | Soluble in ethanol and methan | ||
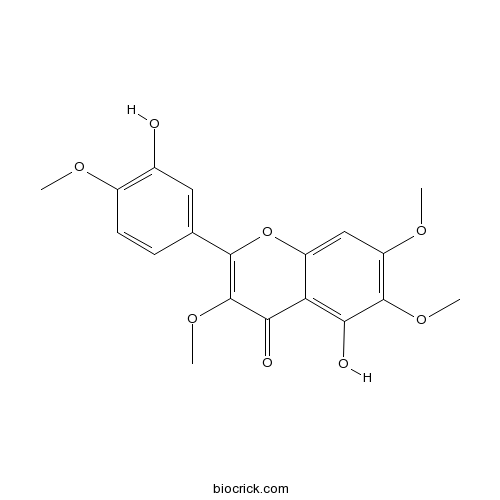
| Chemical Name | 5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3,6,7-trimethoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=C(C(=O)C3=C(C(=C(C=C3O2)OC)OC)O)OC)O | ||
| Standard InChIKey | PJQLSMYMOKWUJG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18O8/c1-23-11-6-5-9(7-10(11)20)17-19(26-4)16(22)14-12(27-17)8-13(24-2)18(25-3)15(14)21/h5-8,20-21H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vitexicarpin has shown antitumor, cytotoxicity, anti-inflammatory, analgesic and immunoregulatory properties.Vitexicarpin can act as a novel angiogenesis inhibitor, it exerts good antiangiogenic effects by inhibiting vascular-endothelial-growth-factor-(VEGF-) induced endothelial cell proliferation, migration, and capillary-like tube formation on matrigel in a dose-dependent manner. It can significantly reduce vascular inflammation, through inhibition of ROS-NF-κB pathway in vascular endothelial cells. |
| Targets | TNF-α | ROS | NF-kB | Bcl-2/Bax | VEGFR | Histamine Receptor |
| In vitro | Vitexicarpin induces apoptosis in human prostate carcinoma PC-3 cells through G2/M phase arrest.[Pubmed: 23464460]Asian Pac J Cancer Prev. 2012;13(12):6369-74.Vitexicarpin (3', 5-dihydroxy-3, 4', 6, 7-tetramethoxyflavone), a polymethoxyflavone isolated from Viticis Fructus (Vitex rotundifolia Linne fil.), has long been used as an anti-inflammatory herb in traditional Chinese medicine. It has also been reported that Vitexicarpin can inhibit the growth of various cancer cells. However, there is no report elucidating its effect on human prostate carcinoma cells. Tracheospasmolytic activity of viteosin-A and vitexicarpin isolated from vitex trifolia.[Pubmed: 12451502]Planta Med. 2002 Nov;68(11):1047-9.
|
| Kinase Assay | Vitexicarpin acts as a novel angiogenesis inhibitor and its target network.[Pubmed: 23476684]Vascular protective role of vitexicarpin isolated from Vitex rotundifolia in human umbilical vein endothelial cells.[Pubmed: 21614554]Inflammation. 2012 Apr;35(2):584-93.Pro-inflammatory cytokines induce injury of endothelial cells caused by increases of adhesion molecules, leading to vascular inflammation and the development of atherosclerosis. Recent pharmacological studies have demonstrated that Vitexicarpin, a flavonoid isolated from Vitex rotundifolia, has anti-inflammatory, antitumor, and analgesic properties. Evid Based Complement Alternat Med. 2013;2013:278405.Vitexicarpin (VIT) isolated from the fruits of Vitex rotundifolia has shown antitumor, anti-inflammatory, and immunoregulatory properties. |
| Structure Identification | J Nat Prod. 2003 Jun;66(6):865-7.Cytotoxic flavone analogues of vitexicarpin, a constituent of the leaves of Vitex negundo.[Pubmed: 12828478 ]
|

Vitexicarpin Dilution Calculator

Vitexicarpin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6714 mL | 13.3568 mL | 26.7137 mL | 53.4274 mL | 66.7842 mL |
| 5 mM | 0.5343 mL | 2.6714 mL | 5.3427 mL | 10.6855 mL | 13.3568 mL |
| 10 mM | 0.2671 mL | 1.3357 mL | 2.6714 mL | 5.3427 mL | 6.6784 mL |
| 50 mM | 0.0534 mL | 0.2671 mL | 0.5343 mL | 1.0685 mL | 1.3357 mL |
| 100 mM | 0.0267 mL | 0.1336 mL | 0.2671 mL | 0.5343 mL | 0.6678 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Artemetin
Catalog No.:BCN5547
CAS No.:479-90-3
- Canthin-6-one
Catalog No.:BCN5546
CAS No.:479-43-6
- Indirubin
Catalog No.:BCN2385
CAS No.:479-41-4
- Cotoin
Catalog No.:BCN5545
CAS No.:479-21-0
- Atranorin
Catalog No.:BCN5544
CAS No.:479-20-9
- Dyphylline
Catalog No.:BCC2297
CAS No.:479-18-5
- Coumestrol
Catalog No.:BCN3949
CAS No.:479-13-0
- Calcium-Sensing Receptor Antagonists I
Catalog No.:BCC1448
CAS No.:478963-79-0
- Angiotensin 1/2 (1-6)
Catalog No.:BCC1036
CAS No.:47896-63-9
- Zopfiellamide A
Catalog No.:BCN1865
CAS No.:478945-64-1
- Kisspeptin 10 (rat)
Catalog No.:BCC6132
CAS No.:478507-53-8
- CP 339818 hydrochloride
Catalog No.:BCC7048
CAS No.:478341-55-8
- Aucubin
Catalog No.:BCN5355
CAS No.:479-98-1
- [Orn8]-Urotensin II
Catalog No.:BCC5793
CAS No.:479065-85-5
- MMPIP hydrochloride
Catalog No.:BCC7528
CAS No.:479077-02-6
- TC OT 39
Catalog No.:BCC7958
CAS No.:479232-57-0
- CNQX disodium salt
Catalog No.:BCC6908
CAS No.:479347-85-8
- NBQX disodium salt
Catalog No.:BCC6907
CAS No.:479347-86-9
- AUDA
Catalog No.:BCC4023
CAS No.:479413-70-2
- 1-O-galloyl-2-O-cinnamoyl-beta-d-glucose
Catalog No.:BCC3967
CAS No.:
- CP-809101
Catalog No.:BCC1498
CAS No.:479683-64-2
- Eleutherol
Catalog No.:BCN8480
CAS No.:480-00-2
- Astragalin
Catalog No.:BCN5549
CAS No.:480-10-4
- Oroxylin A
Catalog No.:BCN5363
CAS No.:480-11-5
Tracheospasmolytic activity of viteosin-A and vitexicarpin isolated from vitex trifolia.[Pubmed:12451502]
Planta Med. 2002 Nov;68(11):1047-9.
The n-hexane extract that has shown activity in the tracheospasmolytic bioassay was fractionated by solvent extraction and from the major active fraction two compounds were isolated and identified as viteosin-A and Vitexicarpin. These compounds blocked spontaneous contraction of isolated male guinea pig trachea induced by histamine; however only Vitexicarpin was active in a model using sensitized guinea pig trachea stimulated by ovalbumin up to minimum dose of 1.3 x 10(-5) M. The result suggests that Vitexicarpin is able to block effects of histamine released from sensitized mast cells possibly by stabilizing the mast cells membrane function.
Vitexicarpin acts as a novel angiogenesis inhibitor and its target network.[Pubmed:23476684]
Evid Based Complement Alternat Med. 2013;2013:278405.
Vitexicarpin (VIT) isolated from the fruits of Vitex rotundifolia has shown antitumor, anti-inflammatory, and immunoregulatory properties. This work is designed to evaluate the antiangiogenic effects of VIT and address the underlying action mechanism of VIT by a network pharmacology approach. The results validated that VIT can act as a novel angiogenesis inhibitor. Firstly, VIT can exert good antiangiogenic effects by inhibiting vascular-endothelial-growth-factor- (VEGF-) induced endothelial cell proliferation, migration, and capillary-like tube formation on matrigel in a dose-dependent manner. Secondly, VIT was also shown to have an antiangiogenic mechanism through inhibition of cell cycle progression and induction of apoptosis. Thirdly, VIT inhibited chorioallantoic membrane angiogenesis as well as tumor angiogenesis in an allograft mouse tumor model. We further addressed VIT's molecular mechanism of antiangiogenic actions using one of our network pharmacology methods named drugCIPHER. Then, we tested some key molecules in the VEGF pathway targeted by VIT and verified the inhibition effects of VIT on AKT and SRC phosphorylation. Taken together, this work not only identifies VIT as a novel potent angiogenesis inhibitor, but also demonstrates that network pharmacology methods can be an effective and promising approach to make discovery and understand the action mechanism of herbal ingredients.
Cytotoxic flavone analogues of vitexicarpin, a constituent of the leaves of Vitex negundo.[Pubmed:12828478]
J Nat Prod. 2003 Jun;66(6):865-7.
Bioassay-guided fractionation of the chloroform-soluble extract of the leaves of Vitex negundo led to the isolation of the known flavone Vitexicarpin (1), which exhibited broad cytotoxicity in a human cancer cell line panel. In an attempt to increase the cytotoxic potency of 1, a series of acylation reactions was performed on this compound to obtain its methylated (2), acetylated (3), and six new acylated (4-9) derivatives. Compound 9, the previously unreported 5,3'-dihexanoyloxy-3,6,7,4'-tetramethoxyflavone, showed comparative cytotoxic potency to compound 1 and was selected for further evaluation. However, this compound was found to be inactive when evaluated in the in vivo hollow fiber assay with Lu1, KB, and LNCaP cells at the highest dose (40 mg/kg/body weight) tested, and in the in vivo P-388 leukemia model (135 mg/kg), using the ip administration route.
Vascular protective role of vitexicarpin isolated from Vitex rotundifolia in human umbilical vein endothelial cells.[Pubmed:21614554]
Inflammation. 2012 Apr;35(2):584-93.
Pro-inflammatory cytokines induce injury of endothelial cells caused by increases of adhesion molecules, leading to vascular inflammation and the development of atherosclerosis. Recent pharmacological studies have demonstrated that Vitexicarpin, a flavonoid isolated from Vitex rotundifolia, has anti-inflammatory, antitumor, and analgesic properties. In this study, we investigated whether Vitexicarpin (5-100 nM) prevented the TNF-alpha-induced vascular inflammation process in human umbilical vein endothelial cells (HUVEC). We found that pretreatment with Vitexicarpin decreased TNF-alpha (10 ng/ml)-induced expression of cell adhesion molecules such as vascular cell adhesion molecule-1, intracellular adhesion molecule-1, and E-selectin as well as matrix metalloproteinase-2 and -9 expression. Preincubation with Vitexicarpin also dose-dependently inhibited TNF-alpha-induced adhesion of HL-60 monocytic cells. Vitexicarpin significantly decreased TNF-alpha-induced intracellular reactive oxygen species (ROS) production. Furthermore, Vitexicarpin suppressed NF-kappaB nuclear translocation and transcriptional activity in TNF-alpha-treated HUVEC. In conclusion, Vitexicarpin significantly reduced vascular inflammation, through inhibition of ROS-NF-kappaB pathway in vascular endothelial cells.
Vitexicarpin induces apoptosis in human prostate carcinoma PC-3 cells through G2/M phase arrest.[Pubmed:23464460]
Asian Pac J Cancer Prev. 2012;13(12):6369-74.
Vitexicarpin (3', 5-dihydroxy-3, 4', 6, 7-tetramethoxyflavone), a polymethoxyflavone isolated from Viticis Fructus (Vitex rotundifolia Linne fil.), has long been used as an anti-inflammatory herb in traditional Chinese medicine. It has also been reported that Vitexicarpin can inhibit the growth of various cancer cells. However, there is no report elucidating its effect on human prostate carcinoma cells. The aim of the present study was to examine the apoptotic induction activity of Vitexicarpin on PC-3 cells and molecular mechanisms involved. MTT studies showed that Vitexicarpin dose-dependently inhibited growth of PC-3 cells with an IC50~28.8 muM. Hoechst 33258 staining further revealed that Vitexicarpin induced apoptotic cell death. The effect of Vitexicarpin on PC-3 cells apoptosis was tested using prodium iodide (PI)/Annexin V-FITC double staining and flow cytometry. The results indicated that Vitexicarpin induction of apoptotic cell death in PC-3 cells was accompanied by cell cycle arrest in the G2/M phase. Furthermore, our study demonstrated that Vitexicarpin induction of PC-3 cell apoptosis was associated with upregulation of the proapoptotic protein Bax, and downregulation of antiapoptotic protein Bcl-2, release of Cytochrome c from mitochondria and decrease in mitochondrial membrane potential. Our findings suggested that Vitexicarpin may become a potential leading drug in the therapy of prostate carcinoma.


