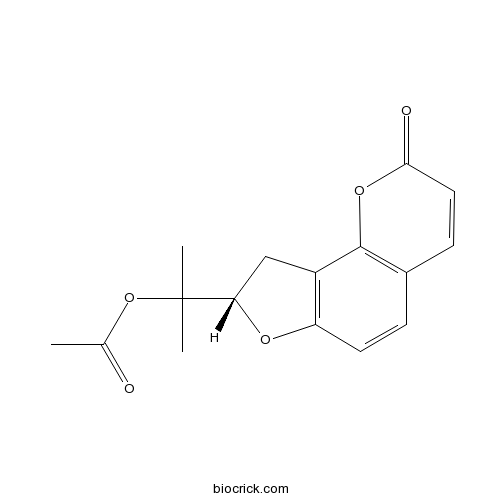
An acetate ester obtained by formal acetylation of the tertiary hydroxy group of 2-[(8S)-2-oxo-8,9-dihydro-2H-furo[2,3-h][1]benzopyran-8-yl]propan-2-ol.
InChI=1S/C16H16O5/c1-9(17)21-16(2,3)13-8-11-12(19-13)6-4-10-5-7-14(18)20-15(10)11/h4-7,13H,8H2,1-3H3/t13-/m0/s1
Columbianetin acetate can be absorbed in whole intestinal sections and colon is the best absorption region of whole rat intestines.The increase of columbianetin acetate concentration has no effect on absorption kinetics,the absorption of columbianetin acetate is a passive diffusion process, not pH-dependent.[1]
Columbianetin may be helpful in regulating mast cell-mediated allergic inflammatory responses.[2]
English website: Columbianetin acetate
Japanese website: Columbianetin acetate
Chinese website: Columbianetin acetate
[1] Wu Y N, Luan L B. Chinese Pharmaceutical Journal, 2008,43(22):1719-22.
[2] Jeong H J, Na H J, Kim S J, et al. Biol Pharml Bull, 2009, 32(6):1027-31.
[3] Cai Q, Sha M, Yang S. Innovation and training in the agribusiness complex, CEDEFOP, European Centre for the Development of Vocational Training. 1999:1580-4.