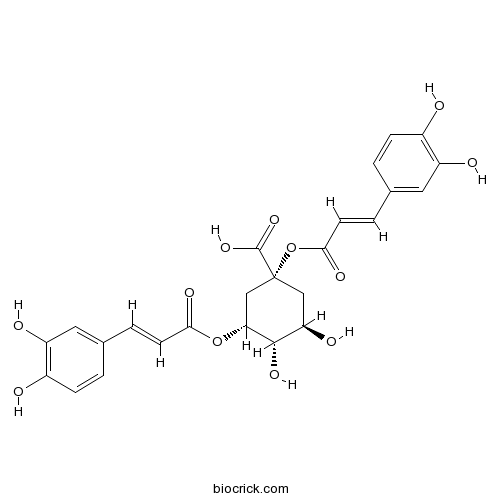
An alkyl caffeate ester obtained by the formal condensation of hydroxy groups at positions 1 and 3 of ()-quinic acid with two molecules of trans-caffeic acid.
1,5-Dicaffeoylquinic acid (1,5-DQA), a caffeoylquinic acid derivative isolated from Aster scaber, has neuroprotective effects, can prevent Aβ(42)-induced neurotoxicity through the activation of PI3K/Akt followed by the stimulation of Trk A, then the inhibition of GSK3β as well as the modulation of Bcl-2/Bax.[1]
1,5-Dicaffeoylquinic acid has antioxidant activity, and is stronger than that of ascorbic acid.[2]
1,5-Dicaffeoylquinic Acid has protective effects against MPP~+ induces neurotoxicity of PC12 Cells, it (50 μmol/L) pretreatment can inhibit the MPP+-induced up-regulation of the expression of α-synuclein mRNA and protein.[3]
1, 5-Dicaffeoylquinic acid can mediate glutathione synthesis through activation of Nrf2 protects against OGD/reperfusion-induced oxidative stress in astrocytes.[4]
English website: 1,5-Dicaffeoylquinic acid
Japanese website: 1,5-Dicaffeoylquinic acid
Chinese website: 1,5-Dicaffeoylquinic acid
[1] XIAO, Hai-bing, WANG, et al. Chinese Medl Jl, 2011, 124(17):2628-35.
[2] Slanina J, Paulová H, Humpa O, et al. Organic Chem, 1999, 72.
[3] Cao X, Xiao H, Li H. Acta Med Universit Sci Et Technol Huazhong, 2010, 39(4):435-38.
[4] Xu C, Xiao H, Zhang Y, et al. Brain Res, 2010, 1347(1):142-8.
[5] Dong Y, Zhang Y, Liu Y, et al. Chinese J Pharm, 2010, 41(6):447-9.