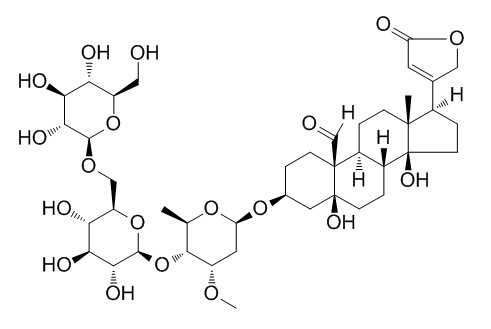
k-StrophanthosideCAS# 33279-57-1 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 33279-57-1 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | White-beige powder |
| Formula | C42H64O19 | M.Wt | 873.0 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | K-Strophanthoside is deterrent to Oviposition responses of Pieris rapae and P. napi oleracea. | |||||

k-Strophanthoside Dilution Calculator

k-Strophanthoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1455 mL | 5.7274 mL | 11.4548 mL | 22.9095 mL | 28.6369 mL |
| 5 mM | 0.2291 mL | 1.1455 mL | 2.291 mL | 4.5819 mL | 5.7274 mL |
| 10 mM | 0.1145 mL | 0.5727 mL | 1.1455 mL | 2.291 mL | 2.8637 mL |
| 50 mM | 0.0229 mL | 0.1145 mL | 0.2291 mL | 0.4582 mL | 0.5727 mL |
| 100 mM | 0.0115 mL | 0.0573 mL | 0.1145 mL | 0.2291 mL | 0.2864 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Longifolene
Catalog No.:BCN0120
CAS No.:475-20-7
- VX-702
Catalog No.:BCN0119
CAS No.:479543-46-9
- Lactupicrin
Catalog No.:BCN0118
CAS No.:65725-11-3
- Tricetinidin chloride
Catalog No.:BCN0117
CAS No.:65618-21-5
- (-)-Carveol
Catalog No.:BCN0116
CAS No.:99-48-9
- Sempervirine nitrate
Catalog No.:BCN0115
CAS No.:17994-15-9
- (+)-Isocorydine hydrochloride
Catalog No.:BCN0114
CAS No.:13552-72-2
- alpha-Pinene oxide
Catalog No.:BCN0113
CAS No.:1686-14-2
- Dehydroascorbic acid
Catalog No.:BCN0112
CAS No.:490-83-5
- Eupatorin-5-methylether
Catalog No.:BCN0111
CAS No.:21764-09-0
- Furfuryl acetate
Catalog No.:BCN0110
CAS No.:623-17-6
- DL-Threonine
Catalog No.:BCN0109
CAS No.:80-68-2
- Gomisin U
Catalog No.:BCN0122
CAS No.:135095-46-4
- Acetoxyvalerenic acid
Catalog No.:BCN0123
CAS No.:84638-55-1
- Vitexin 2''-glucoside
Catalog No.:BCN0124
CAS No.:61360-94-9
- Fortunellin
Catalog No.:BCN0125
CAS No.:20633-93-6
- 3',5'-Dimethoxy-4'-hydroxyacetophenone
Catalog No.:BCN0126
CAS No.:2478-38-8
- Bacosine
Catalog No.:BCN0127
CAS No.:198014-94-7
- Laudanosine
Catalog No.:BCN0128
CAS No.:1699-51-0
- Negundoside
Catalog No.:BCN0129
CAS No.:82451-20-5
- 2,3-Dehydrosilybin A
Catalog No.:BCN0130
CAS No.:25166-14-7
- (-)-Cadin-4,10(15)-dien-11-oic acid
Catalog No.:BCN0131
CAS No.:1124353-23-6
- (+)-Lariciresinol 4'-O-beta-D-Glucopyranosyl-(1->3)-beta-D-glucopyranoside
Catalog No.:BCN0132
CAS No.:639857-95-7
- 3',4',7,8-Tetramethoxyflavone
Catalog No.:BCN0133
CAS No.:65548-55-2
LC-ESI-MS/MS characterization of strophanthin-K.[Pubmed:15907623]
J Pharm Biomed Anal. 2005 Jun 1;38(1):79-86.
A liquid chromatography-mass spectrometry (LC-MS) method was developed for the characterization of strophanthin-K, a mixture of cardiac glycosides extracted from the seeds of Strophanthus kombe. The method is based on the separation of the cardenolides using high performance liquid chromatography (HPLC) followed by detection with electrospray ionization mass-spectrometry (ESI-MS). Chromatographic separation of the analytes was achieved on a RP C-18 column using water: 1% formic acid in water (v/v):acetonitrile gradient mobile phase. Strophanthin-K glycosides studied in ESI-MS in negative ion mode formed abundant adduct ions [M+HCOO](-) while the pseudomolecular ions [M-H](-) are obtained in ESI-MS/MS experiments. Six different cardiac glycosides were identified and characterized: k-Strophanthoside, k-strophanthin-beta, helveticoside (erysimin), erysimoside, cymarin and neoglucoerysimoside. Forced degradation investigations done with strophanthin-K showed that k-strophanthidin (the aglycone of strophanthin-K glycosides) was the main product of degradation in acidic conditions; however, in basic conditions, the hydrolysis of the unsaturated 17beta-lactones to the corresponding gamma-hydroxy acids was the predominant degradation pathway.
Cardenolides as oviposition deterrents to twoPieris species: Structure-activity relationships.[Pubmed:24242301]
J Chem Ecol. 1994 May;20(5):1039-51.
Oviposition responses ofPieris rapae andP. napi oleracea to 18 cardenolides were compared under the same conditions. Effects of different concentrations of selected cardenolides were also tested. Most of the compounds were deterrent to oviposition by both insects, but to significantly different degrees.P. rapae were strongly deterred by k-Strophanthoside, K-strophanthin-beta, cymarin, convallatoxin, oleandrin, erysimoside, erychroside, and gitoxigenin. The most deterrent compounds forP. napi oleracea were erychroside, cymarin, erysimoside, convallatoxin, and k-Strophanthoside. Strophanthidin-based glycosides were more deterrent than digitoxigenin-based ones, and the number and type of sugar substitutions can have profound effects on activity. Both similarities and contrasts were found in responses ofP. rapae andP. napi oleracea to these cardenolides. Cymarin was equally deterrent to bothPieris species at all concentrations tested. However, when compared withP. rapae, P. napi oleracea was less sensitive to most of the cardenolides.P. napi oleracea was insensitive to K-strophanthin-beta and oleandrin at 0.5 x 10(-4) M, which were highly deterrent toP. rapae.
Biodistribution of two 99mTc-cardiac glycosides with end glucose unit: effect of lipophilicity on their relative myocardial accumulation.[Pubmed:2715000]
Int J Rad Appl Instrum B. 1989;16(1):41-6.
In biodistribution experiments with tritium labelled cardiac glycoside it was observed that compounds of low lipophilicity showed a considerably higher affinity towards myocardium with respect to other tissues and organs. A similar trend was also observed with 99mTc-cardiac glycosides except for one compound with glucose residue, which in spite of its lower lipophilicity exhibited an unexpectedly low heart to non-target concentration ratio, thereby indicating a possible influence of carbohydrate residue on biodistribution. To confirm this, in this article we radiolabelled two glucose containing cardiac glycosides (K-strophanthin-beta and k-Strophanthoside) with 99mTc and, in biodistribution experiments, less lipophilic 99mTc-k-Strophanthoside showed a much better heart to non-target ratio over 99mTc-K-strophanthin-beta. It is thus concluded that, in addition to lipophilicity, the affinity of the carbohydrate residue for non-target organs is also an important consideration in determining the structure-distribution relationship of 99mTc-cardiac glycosides.
The action of prostaglandins on ureter smooth muscle of guinea-pig.[Pubmed:2446883]
Eur J Pharmacol. 1987 Oct 6;142(1):163-7.
Prostaglandins of the E type (PGE) relaxed guinea-pig ureter but prostaglandins of the F type (PGF) did not affect smooth muscle contraction. Hyperpolarization and relaxation of the muscle cells caused by the PGEs were achieved at concentrations in a different range, a feature also observed in the presence of forskolin or iso-butyl-methyl-xanthine (IBMX). Hyperpolarization was inhibited in the presence of k-Strophanthoside. The c-AMP content of ureter smooth muscle cells was increased in the presence of PGE2. These observations suggest that the PGE-induced hyperpolarization is caused by activation of the sodium-potassium pump and that enhancement of the cellular c-AMP level plays a major role in the PGE-induced relaxation.
Absorption, metabolism and elimination of strophanthus glycosides in man.[Pubmed:3821940]
Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):496-500.
In 33 healthy male volunteers, given a single oral and intravenous dose of cymarin (k-strophanthin-alpha), k-Strophanthoside (k-strophanthin-gamma) and ouabain (g-strophanthin), enteral absorption and renal excretion of these glycosides and their metabolites were investigated by radioimmunoassay and HPLC. Cymarin was absorbed at 47% of the given dose. After intravenous injection 46% and after oral administration 21% of the given dose, i.e. the total amount as detected by radioimmunoassay which consisted of the unchanged glycoside and its metabolites, were excreted by the kidneys mainly as conjugated metabolites. The half-life of elimination, calculated from the total excreted amount was 13 h (i.v.) and 23 h (p.o.), respectively. k-Strophanthoside was absorbed at 16% of the given dose. After i.v.-injection 73% of the given dose was excreted by the kidneys with a half-life of elimination of 99 h. From this total amount about 70% was excreted as the unchanged drug, the remaining 30% as various metabolites. After oral administration 11% of the given dose were excreted with a half-life of elimination of 22 h. 80% of this amount consisted mainly of conjugated k-Strophanthoside and conjugated metabolites as k-strophanthin-beta, cymarin, k-strophanthidin, cymarol and k-strophanthidol. Only 6% was excreted as the unchanged drug. Ouabain was absorbed after oral administration to a minimum of 1.4%. Given intravenously a total renal excretion of 33% of the given dose with a half-life of elimination of 23 h was measured. Of this 80% was unchanged ouabain.(ABSTRACT TRUNCATED AT 250 WORDS)
Activatory effect of two cardioglycosides on Cavia cobaya kidney Na+/K+-ATPase activity.[Pubmed:2991074]
Gen Pharmacol. 1985;16(3):183-8.
Ouabain and k-Strophanthoside promote an enhancement of Na+/K+-ATPase activity in a range of cardioglycoside concentrations from 100 nM to 100 pM, with a maximum (+30%) between 10 and 4 nM. Binding experiments with [3H]ouabain show upward-curved Scatchard plots and evidence two intrinsic affinity constants for the ligand: (a) High-affinity constant: 350 nM (microsomes) and 15 nM (purified enzyme). (b) Low-affinity constant: 2100 nM (microsomes) and 890 nM (purified enzyme). The reaction velocity trend indicates that at ouabain concentrations higher than 20 nM but lower than the minimal inhibiting level, the enhanced reaction velocity is tending towards the control values.
Impact of neonatal treatment with cardioactive glycosides (digoxin, ouabain) on receptor binding capacity, blood level and cardiac function in the adult rat. Extension of the imprinting theory.[Pubmed:6319227]
Gen Pharmacol. 1983;14(6):709-11.
A single neonatal treatment with a cardioactive glycoside (ouabain, digoxin) altered the response of the adult rat to digitaloid treatment. As demonstrated by RIA, re-exposure to digoxin at 2 months of age was followed within 30 min by a more than twofold increase in serum digoxin over the not pretreated control and, although a steady concentration decrease followed, the experimental rats still had a higher serum digoxin level than the controls at 4 h. In the not presensitized control group the serum digoxin peak appeared at 60 min at a considerably lower level than the 30-min maximum of the experimental rats. Neonatal pretreatment with ouabain depressed myocardial ouabain binding, but enhanced the Na+ K+ -ATPase activity. The above differences were conspicuous in the functional response, yet a greater atrial response to the positive inotropic action of k-Strophanthoside and a greater ventricular response to beta adrenergic excitation were readily seen in the experimental group. It follows that not only hormones, but also other ligands acting at receptor level can be regarded as potential inducers of imprinting.
[Effects of glucosides on a spontaneously active vascular smooth muscle in vitro (author's transl)].[Pubmed:6896365]
Neurochirurgia (Stuttg). 1981 Sep;24(5):170-3.
There is no support for the view that cardiac glucosides increase the resting tension in the rat portal vein, although such an effect on vascular smooth muscle might have been expected from the results of investigation on the coronary vessels. Glucosides in a concentration of 10(-4) m inhibit contractility while increasing the frequency of contractions. These changes of spontaneous activity are not accompanied by equivalent changes of the intracellular cation concentrations. There are no significant differences between the effects of g-strophanthine, k-Strophanthoside and convallatoxin.
Nifedipine for digitalis-induced arrhythmias.[Pubmed:7404089]
S Afr Med J. 1980 Jun 21;57(25):1046.
The calcium antagonist, nifedipine (Adalat; Bayer), has previously been thought by most workers to lack cardiac-anti-arrhythmic properties, thus differing from verapamil. We have found that nifedipine was successful in converting cardiac glycoside (k-Strophanthoside)-induced ventricular tachycardia to sinus rhythm in 4 of 5 anaesthetized dogs when given intravenously. In this respect, therefore, nifedipine is similar to verapamil. Further work is advocated before applying these results to the clinical situation in man.


