1-PhenylethanolCAS# 98-85-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 98-85-1 | SDF | Download SDF |
| PubChem ID | 7409 | Appearance | Oil |
| Formula | C8H10O | M.Wt | 122.2 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-phenylethanol | ||
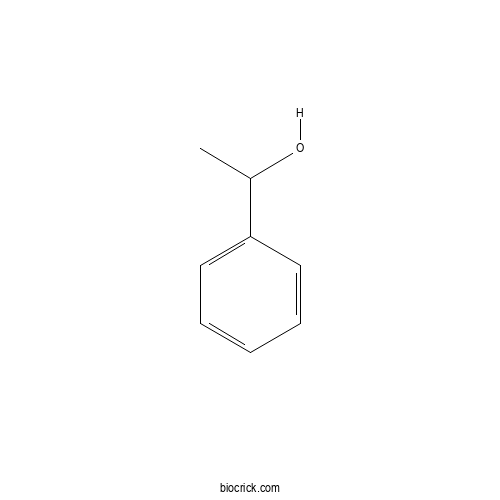
| SMILES | CC(C1=CC=CC=C1)O | ||
| Standard InChIKey | WAPNOHKVXSQRPX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H10O/c1-7(9)8-5-3-2-4-6-8/h2-7,9H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1-Phenylethanol has a nongenotoxic mode of action responsible for the lung and liver tumors observed in mice following 2 years of inhalation exposure to ethylbenzene. | |||||

1-Phenylethanol Dilution Calculator

1-Phenylethanol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.1833 mL | 40.9165 mL | 81.8331 mL | 163.6661 mL | 204.5827 mL |
| 5 mM | 1.6367 mL | 8.1833 mL | 16.3666 mL | 32.7332 mL | 40.9165 mL |
| 10 mM | 0.8183 mL | 4.0917 mL | 8.1833 mL | 16.3666 mL | 20.4583 mL |
| 50 mM | 0.1637 mL | 0.8183 mL | 1.6367 mL | 3.2733 mL | 4.0917 mL |
| 100 mM | 0.0818 mL | 0.4092 mL | 0.8183 mL | 1.6367 mL | 2.0458 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3',4',5'-Trimethoxyflavone
Catalog No.:BCN9943
CAS No.:67858-30-4
- Ethyl salicylate
Catalog No.:BCN9942
CAS No.:118-61-6
- Coniine hydrochloride
Catalog No.:BCN9941
CAS No.:15991-59-0
- Enterodiol
Catalog No.:BCN9940
CAS No.:77756-22-0
- Oleacein
Catalog No.:BCN9939
CAS No.:149183-75-5
- Apioline
Catalog No.:BCN9938
CAS No.:523-80-8
- Cevadine
Catalog No.:BCN9937
CAS No.:62-59-9
- Carvacrol methyl ether
Catalog No.:BCN9936
CAS No.:6379-73-3
- 2,4-Dimethylphenol
Catalog No.:BCN9935
CAS No.:105-67-9
- Nortropinone hydrochloride
Catalog No.:BCN9934
CAS No.:25602-68-0
- Sodium pyruvate
Catalog No.:BCN9933
CAS No.:113-24-6
- 3-Carene
Catalog No.:BCN9932
CAS No.:13466-78-9
- Hyoscyamine hydrobromide
Catalog No.:BCN9945
CAS No.:306-03-6
- 2',4'-Dihydroxydihydrochalcone
Catalog No.:BCN9946
CAS No.:53596-71-7
- Pipermethystine
Catalog No.:BCN9947
CAS No.:71627-22-0
- 2,4-Dihydroxybenzoic acid
Catalog No.:BCN9948
CAS No.:89-86-1
- Petroselinic acid
Catalog No.:BCN9949
CAS No.:593-39-5
- Manassantin B
Catalog No.:BCN9950
CAS No.:88497-88-5
- 4',5,7-Trihydroxy 3,3',6,8-tetramethoxyflavone
Catalog No.:BCN9951
CAS No.:58130-91-9
- 2',5,6',7-Tetrahydroxyflavone
Catalog No.:BCN9952
CAS No.:82475-00-1
- Lapatinib (GW-572016) Ditosylate
Catalog No.:BCN9953
CAS No.:388082-77-7
- Artepillin C
Catalog No.:BCN9954
CAS No.:72944-19-5
- 4-Methylcatechol
Catalog No.:BCN9955
CAS No.:452-86-8
- Isogentisin
Catalog No.:BCN9956
CAS No.:491-64-5
Noncovalently Linked Metallacarboranes on Functionalized Magnetic Nanoparticles as Highly Efficient, Robust, and Reusable Photocatalysts in Aqueous Medium.[Pubmed:33284598]
ACS Appl Mater Interfaces. 2020 Dec 7.
A successful homogeneous photoredox catalyst has been fruitfully heterogenized on magnetic nanoparticles (MNPs) coated with a silica layer, keeping intact its homogeneous catalytic properties but gaining others due to the easy magnetic separation and recyclability. The amine-terminated magnetic silica nanoparticles linked noncovalently to H[3,3'-Co(1,2-C2B9H11)2](-) (H[1]), termed MSNPs-NH2@H[1], are highly stable and do not produce any leakage of the photoredox catalyst H[1] in water. The magnetite MNPs were coated with SiO2 to provide colloidal stability and silanol groups to be tethered to amine-containing units. These were the MSNPs-NH2 on which was anchored, in water, the cobaltabis(dicarbollide) complex H[1] to obtain MSNPs-NH2@H[1]. Both MSNPs-NH2 and MSNPs-NH2@H[1] were evaluated to study the morphology, characterization, and colloidal stability of the MNPs produced. The heterogeneous MSNP-NH2@H[1] system was studied for the photooxidation of alcohols, such as 1-Phenylethanol, 1-hexanol, 1,6-hexanediol, or cyclohexanol among others, using catalyst loads of 0.1 and 0.01 mol %. Surfactants were introduced to prevent the aggregation of MNPs, and cetyl trimethyl ammonium chloride was chosen as a surfactant. This provided adequate stability, without hampering quick magnetic separation. The results proved that the catalysis could be speeded up if aggregation was prevented. The recyclability of the catalytic system was demonstrated by performing 12 runs of the MSNPs-NH2@H[1] system, each one without loss of selectivity and yield. The cobaltabis(dicarbollide) catalyst supported on silica-coated magnetite nanoparticles has proven to be a robust, efficient, and easily reusable system for the photooxidation of alcohols in water, resulting in a green and sustainable heterogeneous catalytic system.
Modular O- vs. N-coordination of pyridylidene amide ligands to iron determines activity in alcohol oxidation catalysis.[Pubmed:33232405]
Dalton Trans. 2020 Nov 24.
A family of polydentate pyridine-substituted pyridylidene amide (PYA) complexes bound to iron(ii) was developed. The variation of the coordination set from NN-bidentate PYA to tridentate pincer-type pyPYA2 systems (pyPYA2 = 2,6-bis(PYA)pyridine) had a large influence on the binding mode to iron(ii), including a change from the N- to rare O-coordination of the PYA site and a concomitant shift of the predominant ligand resonance structure. These binding mode variations invoke changes in the reactivity of the complexes, which were probed in the peroxide-mediated oxidation of 1-Phenylethanol to acetophenone. A comparison with uncomplexed FeCl2 indicated that bidentate NN coordination is unstable and presumably leads to the dissociation of FeCl2. In contrast, the tridentate ligand binding is robust. Remarkably, the tridentate PYA pincer coordination inhibits catalytic activity in the NNN binding mode, while the ONO coordination greatly enhances catalytic performance. Under optimized conditions, the bis-ligated ONO pincer iron complex [Fe(pyPYA2)2][2PF6] reaches full conversion within one hour (0.5 mol% catalyst loading) and under dilute conditions turnover numbers over 20 000 (0.005 mol% catalyst loading).
Iron-Catalyzed Oxidation of 1-Phenylethanol and Glycerol With Hydrogen Peroxide in Water Medium: Effect of the Nitrogen Ligand on Catalytic Activity and Selectivity.[Pubmed:33195031]
Front Chem. 2020 Oct 9;8:810.
The iron(II) complexes [Fe(bpy)3](OTf)2 (bpy = 2,2'-bipyridine; OTf = CF3SO3) (1) and [Fe(bpydeg)3](OTf)2 (bpydeg = N (4),N (4)-bis(2-(2-methoxyethoxy)ethyl) [2,2'-bipyridine]-4,4'-dicarboxamide) (2), the latter being a newly synthesized ligand, were employed as catalyst precursors for the oxidation of 1-Phenylethanol with hydrogen peroxide in water, using either microwave or conventional heating. With the same oxidant and medium the oxidation of glycerol was also explored in the presence of 1 and 2, as well as of two similar iron(II) complexes bearing tridentate ligands, i.e., [Fe(terpy)2](OTf)2 (terpy = 2, 6-di(2-pyridyl)pyridine) (3) and [Fe(bpa)2](OTf)2 (bpa = bis(2-pyridinylmethyl)amine) (4): in most reactions the major product formed was formic acid, although with careful tuning of the experimental conditions significant amounts of dihydroxyacetone were obtained. Addition of heterocyclic amino acids (e.g., picolinic acid) increased the reaction yields of most catalytic reactions. The effect of such additives on the evolution of the catalyst precursors was studied by spectroscopic (NMR, UV-visible) and ESI-MS techniques.
Homogeneous Nickel-Catalyzed Sustainable Synthesis of Quinoline and Quinoxaline under Aerobic Conditions.[Pubmed:33174416]
J Org Chem. 2020 Dec 4;85(23):14971-14979.
Dehydrogenative coupling-based reactions have emerged as an efficient route toward the synthesis of a plethora of heterocyclic rings. Herein, we report an efficacious, nickel-catalyzed synthesis of two important heterocycles such as quinoline and quinoxaline. The catalyst is molecularly defined, is phosphine-free, and can operate at a mild reaction temperature of 80 degrees C. Both the heterocycles can be easily assembled via double dehydrogenative coupling, starting from 2-aminobenzyl alcohol/1-Phenylethanol and diamine/diol, respectively, in a shorter span of reaction time. This environmentally benign synthetic protocol employing an inexpensive catalyst can rival many other transition-metal systems that have been developed for the fabrication of two putative heterocycles. Mechanistically, the dehydrogenation of secondary alcohol follows clean pseudo-first-order kinetics and exhibits a sizable kinetic isotope effect. Intriguingly, this catalyst provides an example of storing the trapped hydrogen in the ligand backbone, avoiding metal-hydride formation. Easy regeneration of the oxidized form of the catalyst under aerobic/O2 oxidation makes this protocol eco-friendly and easy to handle.
Understanding benzyl alcohol aggregation by chiral modification: the pairing step.[Pubmed:33169124]
Phys Chem Chem Phys. 2020 Nov 18;22(44):25538-25551.
A combination of linear infrared and Raman spectroscopy in supersonic slit jet expansions is used to clarify the conformational preferences in the dimer of the transiently chiral benzyl alcohol (phenylmethanol) under vacuum isolation. By experimentally exploring close analogies with the permanently chiral 1-Phenylethanol, which is conformationally locked in the jet through intramolecular chirality induction, and by computational analysis of their conformational energy landscapes, several conclusions are drawn. The lowest energy dimer is confirmed to be cooperatively OHOHpi-bonded and shown to be homochiral. Its heterochiral counterpart is slightly higher in energy and can be spectrally assigned as a minor constituent. A metastable heterochiral OHpi/OHpi structure with weakly coupled hydrogen bonds is efficiently trapped behind a Ci symmetry-enhanced barrier and can be assigned by IR/Raman mutual exclusion. Its homochiral counterpart is kinetically less stable but might be addressed by rotational spectroscopy. Ratings of standard density functionals with a standard basis set in terms of reproducing these experimental chirality synchronization benchmarks are presented.
Catalytic Performance of a Magnetic Core-Shell Iron(II) C-Scorpionate under Unconventional Oxidation Conditions.[Pubmed:33114194]
Nanomaterials (Basel). 2020 Oct 23;10(11). pii: nano10112111.
For the first time, herein is reported the use of a magnetic core-shell support for a C-scorpionate metallic complex. The prepared hybrid material, that consists on the C-scorpionate iron(II) complex [FeCl2{kappa(3)-HC(pz)3}] (pz, pyrazolyl) immobilized at magnetic core-shell particles (Fe3O4/TiO2), was tested as catalyst for the oxidation of secondary alcohols using the model substrate 1-Phenylethanol. Moreover, the application of alternative energy sources (e.g., ultrasounds, microwaves, mechanical or thermal) for the peroxidative alcohol oxidation using the magnetic heterogenized iron(II) scorpionate led to different/unusual outcomes that are presented and discussed.
Immobilized Arabidopsis thaliana Hydroxynitrile Lyase-Catalyzed Retro-Henry Reaction in the Synthesis of (S)-beta-Nitroalcohols.[Pubmed:33044692]
Appl Biochem Biotechnol. 2020 Oct 12. pii: 10.1007/s12010-020-03442-3.
Enantiopure beta-nitroalcohols are versatile intermediates used in the synthesis of important pharmaceuticals and chiral synthons. In this article, immobilized Arabidopsis thaliana HNL (AtHNL)-catalyzed preparation of (S)-beta-nitroalcohols from their racemic mixtures via retro-Henry reaction was studied. AtHNL used in biocatalysis was immobilized by physical adsorption in inexpensive celite(R)545. Under optimized biocatalytic conditions, the total turnover number of the catalyst has improved 2.3-fold for (S)-2-nitro-1-Phenylethanol (NPE) synthesis, than free enzyme catalysis. This study reported for the first time celite-AtHNL-catalyzed retro-Henry reaction at low pH. At pH 4.5 and 5.0, 62% ee and 41% conversion, and 97% ee and 42% conversion of (S)-NPE were obtained respectively, while the free enzyme inactivates at pH < 5.0. The increased catalytic efficiency and pH stability of the catalyst could be possibly due to increased stability of AtHNL by immobilization. A dozen of racemic beta-nitroalcohols were converted into their corresponding (S)-beta-nitroalcohols using this reaction; among them, eight were not tested earlier. The immobilized enzyme has showed broad substrate selectivity in the retro-Henry reaction, and products were obtained up to 98.5% ee.
Potential Inhibitors of Protein Tyrosine Phosphatase (PTP1B) Enzyme: Promising Target for Type-II Diabetes Mellitus.[Pubmed:32888269]
Curr Top Med Chem. 2020;20(29):2692-2707.
BACKGROUND: There has been growing interest in the development of highly potent and selective protein tyrosine phosphatase (PTP1B) inhibitors for the past 2-3 decades. Though most PTPs share a common active site motif, the interest in selective inhibitors, particularly against PTP1B is increasing to discover new chemical entities as antidiabetic agents. In the current paradigm to find potent and selective PTP1B inhibitors, which is currently considered as one of the best validated biological targets for non-insulin-dependent diabetic and obese individuals, resistance to insulin due to decreased sensitivity of the insulin receptor is a pathological factor and is also genetically linked, causing type II diabetes. OBJECTIVE: Insulin receptor sensitization is performed by a signal transduction mechanism via a selective protein tyrosine phosphatase (PTP1B). After the interaction of insulin with its receptor, autophosphorylation of the intracellular part of the receptor takes place, turning it into an active kinase (sensitization). PTP1B is involved in the desensitization of the receptor by dephosphorylation. PTP1b inhibitors delay the receptor desensitization, prolonging insulin effect and making PTP1B as a drug target for the treatment of diabetes II. Therefore, it has become a major target for the discovery of potent drugs for the treatment of type II diabetes and obesity. An attempt has been made in the present study to discuss the latest design and discovery of protein tyrosine phosphatase (PTP1B) inhibitors. METHODS: Many PTP1B inhibitors such as diaminopyrroloquinazoline, triazines, pyrimido triazine derivatives, 2-(benzylamino)-1-Phenylethanol, urea, acetamides and piperazinylpropanols, phenylsulphonamides and phenylcarboxamide, benzamido, arylcarboxylic acid derivatives, arylsupfonyl derivatives, thiazoles, isothiozolidiones and thiazolodinones have been discussed, citing the disease mechanisms. RESULTS: The reader will gain an overview of the structure and biological activity of recently developed PTPs inhibitors. CONCLUSION: The co-crystallized ligands and the screened inhibitors could be used as a template for the further design of potent congeners.
Mechanism and Origin of Chemoselectivity of Ru-Catalyzed Cross-Coupling of Secondary Alcohols to beta-Disubstituted Ketones.[Pubmed:32865421]
J Org Chem. 2020 Oct 2;85(19):12444-12455.
Ru-catalyzed cross-coupling of secondary alcohols with only byproducts H2 and H2O provides a green synthetic strategy to prepare beta-disubstituted ketones. Density functional theory (DFT) calculations were performed with the coupling of 1-Phenylethanol and cyclohexanol as a model reaction to gain deeper mechanistic insights herein. The mechanistic details of the main reaction and the key steps of possible side reactions were clarified, and the obtained results are consistent with reported selectivity. Hydrogenation of alpha,beta-unsaturated ketones and dehydrogenation of ruthenium hydride intermediate are direct chemoselectivity-determining stages. The hydrogenation via 1,4-addition generates more stable intermediates, being favored over that via 1,2-addition, and thus avoids the formation of alkene products. The conjugation and pi-pi stacking effects of phenyl and the weak electronic effect of alkyls explain the dominance of specific ketone products in the hydrogenation stage. Hydrogenation of ketone products is kinetically operative but not exergonic enough to stop the irreversible dihydrogen release in an open reaction system, and thus alcohol products are absent. Furthermore, water evaporation in aldol condensation is found to be a double-edged sword, as it can accelerate the hydrogenation stage to prevent alpha,beta-unsaturated ketones from being the main products but decrease the selectivity therein from thermodynamics overall.
Phosphorescent Iridium(III) Complexes with a Dianionic C,C',N,N'-Tetradentate Ligand.[Pubmed:32856908]
Inorg Chem. 2020 Sep 8;59(17):12286-12294.
To prepare new phosphorescent iridium(III) emitters, 2-phenyl-6-(1-phenyl-1-(pyridin-2-yl)ethyl)pyridine (H2L) has been designed and its reactions with [Ir(mu-Cl)(eta(4)-COD)]2 (1, COD = 1,5-cyclooctadiene) have been studied. The products obtained depend on the refluxing temperature of the solvent. Thus, complexes Ir(kappa(4)-C,C',N,N'-L)Cl(CO) (2), [Ir(eta(4)-COD)(kappa(2)-N,N'-H2L)][IrCl2(eta(4)-COD)] (3), and [Ir(mu-Cl)(kappa(4)-C,C',N,N'-L)]2 (4) have been formed in 2-ethoxyethanol, propan-2-ol, and 1-Phenylethanol, respectively. Complex 4 reacts with K(acac) to give the acetylacetonate derivative Ir(kappa(4)-C,C',N,N'-L)(acac) (5). Complexes 2 and 5 are efficient blue-green and green emitters of classes [6tt+1m+2m] and [6tt+3b], respectively. They display lifetimes in the range of 1.1-4.5 mus and high quantum yields (0.54-0.87) in both PMMA films and 2-MeTHF at room temperature.
N-Heterocyclic Carbene as a Surface Platform for Assembly of Homochiral Metal-Organic Framework Thin Films in Chiral Sensing.[Pubmed:32846477]
ACS Appl Mater Interfaces. 2020 Aug 26;12(34):38357-38364.
N-heterocyclic carbenes (NHCs) have attracted increasing attention on surface assembly due to their strong metal binding property, but an NHC-modified metal surface as a new growth platform to assemble other functional materials is still a challenge. Here, we report the preparation and chiral sensing properties of homochiral metal-organic framework thin films on carboxyl-containing NHC self-assembled monolayer-modified gold (Au(NHC)) substrates. By using a liquid-phase epitaxial layer-by-layer method, enantiopure [Cu2(cam)2dabco]n thin films with preferred [110] crystal orientation have been successfully grown on Au(NHC) surfaces. The results of electrochemical cyclic voltammetry and quartz crystal microbalance uptakes of (R)- and (S)-1-Phenylethanol show that the chiral porous thin film on the robust Au(NHC) surface has good enantiomeric electrochemical recognition and enantioselective adsorption. The present work is a new step to develop metal-NHCs as surface platforms for the preparation of multifunctional thin films for sensing applications.
Self-Assembly of Peptide Chiral Nanostructures with Sequence-Encoded Enantioseparation Capability.[Pubmed:32787008]
Langmuir. 2020 Sep 8;36(35):10361-10370.
Biopolymers such as polysaccharides and proteins have been widely used for the chiral separation of various components due to the intrinsic chirality of the polymers. Amyloid-like short peptides can also self-assemble into diverse chiral supramolecular nanostructures or polymers with precisely tailored architectures driving by noncovalent interactions. However, the use of such supramolecular nanostructures for the resolution and separation of chiral components remains largely unexplored. Here, we report that the self-assembled peptide supramolecular nanostructures can be used for the highly efficient chiral separation of various enantiomers. By rationally designing the constituent amino acid sequence of the peptides and the self-assembling environment, we can fabricate supramolecular polymers with distinct surface charges and architectures, including nanohelices, nanoribbons, nanosheets, nanofibrils, and nanospheres. The various supramolecular nanostructures were then used to resolve the racemic mixtures of alpha-methylbenzylamine, 2-phenylpropionic acid, and 1-Phenylethanol. The results indicated that the self-assembled peptide polymers showed excellent enantioselective separation efficiency for different chiral molecules. The enantioselective separation efficiency of the peptide nanostructures can be tailored by changing their surface charges, morphology, and the constituent amino acid sequences of the peptides.
A novel diclofenac-hydrogel conjugate system for intraarticular sustained release: Development of 2-pyridylamino-substituted 1-phenylethanol (PAPE) and its derivatives as tunable traceless linkers.[Pubmed:32535069]
Int J Pharm. 2020 Jul 30;585:119519.
A local sustained-release drug delivery system, or depot, for intra-articular injection offers the opportunity to release a therapeutic agent directly to the joint with limited need for reinjection. A successful system would provide more consistent efficacy and minimize systemic side effects. In this paper, we explore the potential use of diclofenac, a non-steroidal anti-inflammatory drug, for use in a polymer-conjugate depot system. During the course of our exploration it was determined that "conventional ester" conjugates of diclofenac were not appropriate as upon incubation in buffer (pH 7.4) or in bovine synovial fluid, a considerable amount of undesired diclofenac-lactam was released. Thus we developed a novel linker system for diclofenac in order to minimize the production of the lactam. This new linker enables a diclofenac conjugate system with tunable release rates and minimizes the production of undesired lactam side-products.
Non-peptide molecules in the pedicellariae of Toxopneustes roseus.[Pubmed:32522618]
Toxicon. 2020 Sep;184:143-151.
Toxopneustes roseus is a species of sea urchin with a wide distribution along the eastern Pacific coast. It belongs to the Toxopneustidae family and, like its members, has well-developed globiferous pedicellariae that exert a variety of pharmacological actions. We identified six volatile non-peptide molecules from its globiferous pedicellariae by using GC-MS and RP-HPLC-MS/MS, including: benzoic acid; 2-aminoethanol (MEA); 2-(dimethylamine) ethanol (DMAE); 1- (4-bromophenyl)-1-Phenylethanol (BPPE); 2-[1-(4-bromophenyl)-1- phenylethoxy]-N,N-dimethylethanamine (EMB); and 2-[1-(4-chlorphenyl)-1- phenylethoxy]-N,N-dimethylethanamine (CLX). The construction of a pharmacophore model and the in silico molecular docking of EMB and CLX into the human voltage-gated sodium channel hNaV1.7 allowed establishing that these molecules are structurally similar to local anesthetics and other NaV channel blockers and can bind to the same site receptor in NaV channels; suggesting that both molecules are active components in T. roseus venom. Furthermore, a viable endogenous biopathway is proposed in which T. roseus can synthesize EMB and CLX from benzoic acid, MEA, DMAE, and BPPE as their precursors, which would emphasize the importance of these molecules in the metabolism of this sea urchin.
Aroylhydrazone Schiff Base Derived Cu(II) and V(V) Complexes: Efficient Catalysts towards Neat Microwave-Assisted Oxidation of Alcohols.[Pubmed:32325701]
Int J Mol Sci. 2020 Apr 18;21(8). pii: ijms21082832.
A new hexa-nuclear Cu(II) complex [Cu3(mu2-1kappaNO(2),2kappaNO(2)-L)(mu-Cl)2(Cl)(MeOH)(DMF)2]2 (1), where H4L = N'(1),N'(2)-bis(2-hydroxybenzylidene)oxalohydrazide, was synthesized and fully characterized by IR spectroscopy, ESI-MS, elemental analysis, and single crystal X-ray diffraction. Complex 1 and the dinuclear oxidovanadium(V) one [{VO(OEt)(EtOH)}2(1kappaNO(2),2kappaNO(2)-L)].2H2O (2) were used as catalyst precursors for the neat oxidation of primary (cinnamyl alcohol) and secondary (1-phenyl ethanol, benzhydrol) benzyl alcohols and of the secondary aliphatic alcohol cyclohexanol, under microwave irradiation using tert-butyl hydroperoxide (TBHP) as oxidant. Oxidations proceed via radical mechanisms. The copper(II) compound 1 exhibited higher catalytic activity than the vanadium(V) complex 2 for all the tested alcohol substrates. The highest conversion was found for 1-Phenylethanol, yielding 95.3% of acetophenone in the presence of 1 and in solvent and promoter-free conditions. This new Cu(II) complex was found to exhibit higher activity under milder reaction conditions than the reported aroylhydrazone Cu(II) analogues.


