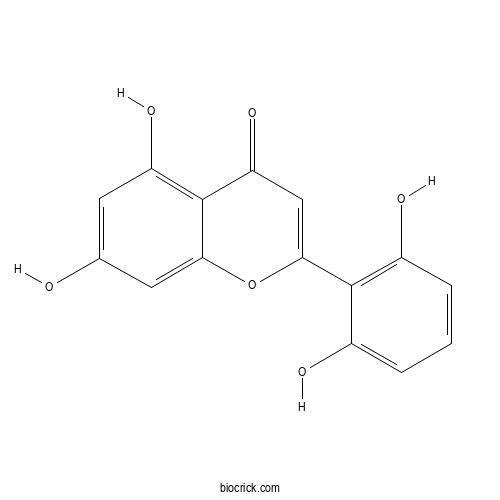
2',5,6',7-TetrahydroxyflavoneCAS# 82475-00-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 82475-00-1 | SDF | Download SDF |
| PubChem ID | 5321865 | Appearance | Powder |
| Formula | C15H10O6 | M.Wt | 286.2 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(2,6-dihydroxyphenyl)-5,7-dihydroxychromen-4-one | ||
| SMILES | C1=CC(=C(C(=C1)O)C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O | ||
| Standard InChIKey | WJXXUIYFPVIHDH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O6/c16-7-4-10(19)15-11(20)6-13(21-12(15)5-7)14-8(17)2-1-3-9(14)18/h1-6,16-19H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2',5,6',7-Tetrahydroxyflavone inhibited hepatic testosterone 6beta-hydroxylation (CYP3A4) activity with a IC50 of 7.8 microM. | |||||

2',5,6',7-Tetrahydroxyflavone Dilution Calculator

2',5,6',7-Tetrahydroxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4941 mL | 17.4703 mL | 34.9406 mL | 69.8812 mL | 87.3515 mL |
| 5 mM | 0.6988 mL | 3.4941 mL | 6.9881 mL | 13.9762 mL | 17.4703 mL |
| 10 mM | 0.3494 mL | 1.747 mL | 3.4941 mL | 6.9881 mL | 8.7352 mL |
| 50 mM | 0.0699 mL | 0.3494 mL | 0.6988 mL | 1.3976 mL | 1.747 mL |
| 100 mM | 0.0349 mL | 0.1747 mL | 0.3494 mL | 0.6988 mL | 0.8735 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4',5,7-Trihydroxy 3,3',6,8-tetramethoxyflavone
Catalog No.:BCN9951
CAS No.:58130-91-9
- Manassantin B
Catalog No.:BCN9950
CAS No.:88497-88-5
- Petroselinic acid
Catalog No.:BCN9949
CAS No.:593-39-5
- 2,4-Dihydroxybenzoic acid
Catalog No.:BCN9948
CAS No.:89-86-1
- Pipermethystine
Catalog No.:BCN9947
CAS No.:71627-22-0
- 2',4'-Dihydroxydihydrochalcone
Catalog No.:BCN9946
CAS No.:53596-71-7
- Hyoscyamine hydrobromide
Catalog No.:BCN9945
CAS No.:306-03-6
- 1-Phenylethanol
Catalog No.:BCN9944
CAS No.:98-85-1
- 3',4',5'-Trimethoxyflavone
Catalog No.:BCN9943
CAS No.:67858-30-4
- Ethyl salicylate
Catalog No.:BCN9942
CAS No.:118-61-6
- Coniine hydrochloride
Catalog No.:BCN9941
CAS No.:15991-59-0
- Enterodiol
Catalog No.:BCN9940
CAS No.:77756-22-0
- Lapatinib (GW-572016) Ditosylate
Catalog No.:BCN9953
CAS No.:388082-77-7
- Artepillin C
Catalog No.:BCN9954
CAS No.:72944-19-5
- 4-Methylcatechol
Catalog No.:BCN9955
CAS No.:452-86-8
- Isogentisin
Catalog No.:BCN9956
CAS No.:491-64-5
- p-Mentha-1,4-diene
Catalog No.:BCN9957
CAS No.:99-85-4
- Citronellyl acetate
Catalog No.:BCN9958
CAS No.:150-84-5
- Withanone
Catalog No.:BCN9959
CAS No.:27570-38-3
- 2',6'-Dihydroxy 4'-methoxydihydrochalcone
Catalog No.:BCN9960
CAS No.:35241-55-5
- Oleocanthal
Catalog No.:BCN9961
CAS No.:289030-99-5
- Aegineoside
Catalog No.:BCN9962
CAS No.:752209-48-6
- 4'-Methylchrysoeriol
Catalog No.:BCN9963
CAS No.:4712-12-3
- 2,3-Dehydrosilybin B
Catalog No.:BCN9964
CAS No.:142796-24-5
Skullcapflavone I from Scutellaria baicalensis induces apoptosis in activated rat hepatic stellate cells.[Pubmed:16206047]
Planta Med. 2005 Sep;71(9):885-7.
The therapeutic goal in liver fibrosis is the reversal of fibrosis and the selective clearance by apoptosis of hepatic stellate cells (HSCs), which play a central role in liver fibrogenesis. In this study, the apoptotic effect of wogonin, oroxylin A, 2',5,6',7-tetrahydroxyflavone, skullcapflavone I, and baicalein, isolated from the dried root of Scutellaria baicalensis, was investigated in activated rat HSCs, T-HSC/Cl-6 cells transformed with the Simian virus 40. Among the isolated compounds, skullcapflavone I (20 microM for 24 h) significantly induced apoptosis in activated rat HSCs while there was no change in the cell viability of hepatocytes. Skullcapflavone I increased caspase-3 and -9 activities accompanied by the proteolytic cleavage of poly(ADP-ribose) polymerase. Specific inhibitors of caspase-3 and caspase-9 prevented the apoptotic process induced by skullcapflavone I. From these results, skullcapflavone I from S. baicalensis selectively induced apoptosis in T-HSC/Cl-6 cells via caspase-3 and caspase-9 activation.
Effects of flavonoids isolated from Scutellariae radix on cytochrome P-450 activities in human liver microsomes.[Pubmed:11936218]
J Toxicol Environ Health A. 2002 Mar;65(5-6):373-81.
A series of flavonoids isolated from Scutellariae radix were evaluated for their effects on cytochrome P-450 (CYP) activities in human liver microsomes. All flavonoids did not substantially inhibit pentoxyresorufin O-deethylation (CYP2B 1), mephenytoin 4-hydroxylation (CYP2C19), dextromethorphan O-demethylation (CYP2D6), and chlorzoxazone 6-hydroxylation (CYP2E1) activities (IC50: >50 microM). Baicalein and 2',5,6',7-tetrahydroxyflavone inhibited hepatic testosterone 6beta-hydroxylation (CYP3A4) activity with a IC50 of 17.4 and 7.8 microM, respectively. Oroxylin A inhibited diclofenac 4-hydroxylation (CYP2C9) activity with a IC50 of 6.7 microM. In contrast, all flavonoids tested inhibited hepatic caffeine N'-demethylation (CYP1A2) with IC50 values ranging from 0.7 to 51.3 microM. Kinetic analysis revealed that the mechanism of inhibition varied according to the flavonoids. These results suggest that flavonoids tested are inhibitors of hepatic CYP1A2 and that the extracts of Scutellariae radix, widely used as a hepatoprotective agent, may protect the liver through the prevention of CYPIA2-induced metabolic activation of protoxicants.
[Studies on the structures of new flavonoids from the root of Scutellaria amoena].[Pubmed:2816376]
Yao Xue Xue Bao. 1989;24(3):200-6.
From the root of Scutellaria amoena C.H. Wright, two new flavonoids (I, II) and six known flavonoids (III-VIII) were isolated. On the basis of spectroscopic analysis (UV, 1HNMR, 13CNMR, MS and CD) and chemical evidences, the structures of I and II were elucidated as (2S)-2',5,6'-trihydroxy-7-methoxyflavanone-2'-O-beta-D-glucopyrano side (I) and (2R, 3R)-2',3,5,7-tetrahydroxyflavanone (II) respectively. The other six known compounds were identified as (2S)-5,7,8-trihydroxyflavanone (III), (2S)-2',5,6',7-tetrahydroxyflavanone (IV), (2R, 3R)-2',3,5,6',7-pentahydroxyflavanone (V), 2',5,6',7-tetrahydroxyflavone (VI) norwogonin (VII) and oroxylin-A (VIII) respectively. Compounds III-VIII were obtained from this plant for the first time.


