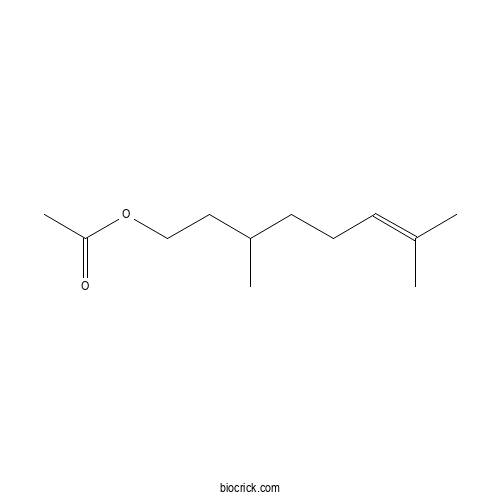
Citronellyl acetateCAS# 150-84-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 150-84-5 | SDF | Download SDF |
| PubChem ID | 9017 | Appearance | Oil |
| Formula | C12H22O2 | M.Wt | 198.3 |
| Type of Compound | Monoterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,7-dimethyloct-6-enyl acetate | ||
| SMILES | CC(CCC=C(C)C)CCOC(=O)C | ||
| Standard InChIKey | JOZKFWLRHCDGJA-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H22O2/c1-10(2)6-5-7-11(3)8-9-14-12(4)13/h6,11H,5,7-9H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Citronellyl acetate(CAT) shows antinociceptive properties on acute pain and shows that, at least in part, TRPV1, TRPM8, ASIC, glutamate receptors, PKC and PKA participate in CAT’s antinociceptive mechanism. | |||||

Citronellyl acetate Dilution Calculator

Citronellyl acetate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0429 mL | 25.2143 mL | 50.4286 mL | 100.8573 mL | 126.0716 mL |
| 5 mM | 1.0086 mL | 5.0429 mL | 10.0857 mL | 20.1715 mL | 25.2143 mL |
| 10 mM | 0.5043 mL | 2.5214 mL | 5.0429 mL | 10.0857 mL | 12.6072 mL |
| 50 mM | 0.1009 mL | 0.5043 mL | 1.0086 mL | 2.0171 mL | 2.5214 mL |
| 100 mM | 0.0504 mL | 0.2521 mL | 0.5043 mL | 1.0086 mL | 1.2607 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- p-Mentha-1,4-diene
Catalog No.:BCN9957
CAS No.:99-85-4
- Isogentisin
Catalog No.:BCN9956
CAS No.:491-64-5
- 4-Methylcatechol
Catalog No.:BCN9955
CAS No.:452-86-8
- Artepillin C
Catalog No.:BCN9954
CAS No.:72944-19-5
- Lapatinib (GW-572016) Ditosylate
Catalog No.:BCN9953
CAS No.:388082-77-7
- 2',5,6',7-Tetrahydroxyflavone
Catalog No.:BCN9952
CAS No.:82475-00-1
- 4',5,7-Trihydroxy 3,3',6,8-tetramethoxyflavone
Catalog No.:BCN9951
CAS No.:58130-91-9
- Manassantin B
Catalog No.:BCN9950
CAS No.:88497-88-5
- Petroselinic acid
Catalog No.:BCN9949
CAS No.:593-39-5
- 2,4-Dihydroxybenzoic acid
Catalog No.:BCN9948
CAS No.:89-86-1
- Pipermethystine
Catalog No.:BCN9947
CAS No.:71627-22-0
- 2',4'-Dihydroxydihydrochalcone
Catalog No.:BCN9946
CAS No.:53596-71-7
- Withanone
Catalog No.:BCN9959
CAS No.:27570-38-3
- 2',6'-Dihydroxy 4'-methoxydihydrochalcone
Catalog No.:BCN9960
CAS No.:35241-55-5
- Oleocanthal
Catalog No.:BCN9961
CAS No.:289030-99-5
- Aegineoside
Catalog No.:BCN9962
CAS No.:752209-48-6
- 4'-Methylchrysoeriol
Catalog No.:BCN9963
CAS No.:4712-12-3
- 2,3-Dehydrosilybin B
Catalog No.:BCN9964
CAS No.:142796-24-5
- Cubebin
Catalog No.:BCN9965
CAS No.:1242843-00-0
- Oxyacanthine sulfate
Catalog No.:BCN9966
CAS No.:6183-91-1
- Genistein 7-O-glucuronide
Catalog No.:BCN9967
CAS No.:38482-81-4
- 4-Ethoxycoumarin
Catalog No.:BCN9968
CAS No.:35817-27-7
- Psoromic acid
Catalog No.:BCN9969
CAS No.:7299-11-8
- Butyric acid
Catalog No.:BCN9970
CAS No.:107-92-6
Modulatory-antibiotic activity of the essential oil from Eucalyptus citriodora against MDR bacterial strains.[Pubmed:32583772]
Cell Mol Biol (Noisy-le-grand). 2020 Jun 25;66(4):60-64.
The growing number of bacterial strains resistant to therapeutic agents has been surpassing the various antibiotics developed by the chemical and pharmaceutical industries. This problem has driven the development of research using agents with antimicrobial potential, with an emphasis on plant-derived natural products. This study evaluated the chemical compounds present in Eucalyptus citriodora essential oil (EOEc) cultivated in northeastern Brazil and its properties as an antibacterial agent and resistance modifier against methicillin-resistant Staphylococcus aureus (MRSA) and beta-lactamase-producing strains. The EOEc was obtained using the hydrodistillation method, later analyzed by GC/MS, presenting a total of twelve compounds, with citronellal (65.45%); citronellol (14.87%); isopulegol (11.80%) and Citronellyl acetate (2.51%) as its main constituents. The microdilution test was used to determine the minimum inhibitory concentration (MIC) and the bacterial resistance modulation of the essential oil. The EOEc did not present significant activity against the tested strains (MIC > 1000 microg mL-1). However, when evaluating the capacity of the EOEc to modify the resistance of S. aureus and E. coli strains to different antimicrobials, synergistic effects were obtained with reduced MIC values for all tested antibiotics being obtained. The EOEc showed antimicrobial and beta-lactam optimizing potential against resistant strains, presenting itself as a possible alternative for the use of these drugs at concentrations lower than those indicated against resistant strains.
Quantitative Structure-Activity Relationship Modeling and Docking of Monoterpenes with Insecticidal Activity Against Reticulitermes chinensis Snyder and Drosophila melanogaster.[Pubmed:32251592]
J Agric Food Chem. 2020 Apr 22;68(16):4687-4698.
The goal of this study was to perform in silico identification of bioinsecticidal potential of 42 monoterpenes against Drosophila melanogaster and Reticulitermes chinensis Snyder. Quantitative structure-activity relationship (QSAR) modeling was performed for both organisms, while docking and molecular dynamics were used only for Drosophila melanogaster. Neryl acetate has the lowest interaction energy (-87 kcal/mol) against active site of acetylcholinesterase, which is comparable to the ones of methiocarb and pirimicarb (-90 kcal/mol) and reported PDB binder 9-(3-iodobenzylamino)-1,2,3,4-tetrahydroacridine (-112.67 kcal/mol). Interaction stability was verified by molecular dynamics simulations and showed that the stability of ACHE active site complexes with three selected terpenes is comparable to the one of the pirimicarb and methiocarb. Overall, our results suggest that pulegone, citronellal, carvacrol, linalyl acetate, neryl acetate, Citronellyl acetate, and geranyl acetate may be considered as a potential pesticide candidates.
Fate of Grape-Derived Terpenoids in Model Systems Containing Active Yeast Cells.[Pubmed:32153191]
J Agric Food Chem. 2020 Nov 25;68(47):13294-13301.
Terpenes are important contributors to wine aroma. Free and glycosidically bound terpenes are primarily formed in grapes. During fermentation, they undergo important transformation catalyzed by yeast, so that the terpene profile of grape is substantially different from that of the corresponding wine. The present paper assessed the ability of a Saccharomyces cerevisiae strain to transform 17 different terpenes. Biotransformation was performed by placing target compounds in incubation with resting cells. Volatile compounds produced were extracted by solid-phase extraction and analyzed by gas chromatography-mass spectrometry. Geranyl acetate, neryl acetate, Citronellyl acetate, and menthyl acetate were formed from the corresponding terpene alcohols. beta-Citronellol was the main product of geraniol transformation; geranial, an intermediate of this pathway, has also been detected. Limonene was hydroxylated by yeast to form carveol, trans-2,8-menthadien-1-ol, and cis-2,8-menthadien-1-ol. Moreover, yeast cells were found to be able to adsorb a significant portion of the terpenes present in the reaction batches, with the extent of this phenomenon being linked to terpene hydrophobicity.
Identification of Floral Scent Profiles in Bearded Irises.[Pubmed:31067789]
Molecules. 2019 May 7;24(9). pii: molecules24091773.
Bearded irises are ornamental plants with distinctive floral fragrance grown worldwide. To identify the floral scent profiles, twenty-seven accessions derived from three bearded iris, including Iris. germanica, I. pumila and I. pallida were used to investigate the composition and relative contents of floral scent components by headspace solid-phase microextraction (HS-SPME) and gas chromatography-mass spectrometry (GC-MS). A total of 219 floral scent components were detected in blooming flowers. The scent profile varied significantly among and within the three investigated species. Principal component analysis (PCA) indicated that terpenes, alcohols and esters contributed the most to the floral scent components and 1-caryophyllene, linalool, citronellol, methyl cinnamate, beta-cedrene, thujopsene, methyl myristate, linalyl acetate, isosafrole, nerol, geraniol were identified as the major components. In a hierarchical cluster analysis, twenty-seven accessions could be clustered into six different groups, most of which had representative scent components such as linalool, Citronellyl acetate, thujopsene, citronellol, methyl cinnamate and 1-caryophyllene. Our findings provide a theoretical reference for floral scent evaluation and breeding of bearded irises.
Olfactory impact of esters on rose essential oil floral alcohol aroma expression in model solution.[Pubmed:30716939]
Food Res Int. 2019 Feb;116:211-222.
This study focused on the impact of esters on the perception of floral aroma in rose essential oil. Various aromatic reconstitutions were prepared, consisting of 10 alcohols and 9 esters, all the concentrations found in rose essential oil. Sensory analysis by the triangular tests revealed the interesting behavior of certain compounds among the 9 esters following their addition or omission. The results tend to highlight the important role of ethyl octanoate, ethyl tetradecanoate, Citronellyl acetate, geranyl acetate, and 2-phenethyl acetate of esters in rose essential oil. The "olfactory threshold" (OT) of the 5 esters, the floral reconstitution and the mixtures of ester and floral reconstitution were evaluated in alkanes solution. Through the Feller's additive model analysis, it was found that the presence of ethyl octanoate, ethyl tetradecanoate, and Citronellyl acetate led to a significant in decrease the OT of the mixtures, whereas geranyl acetate raised the OT. The floral reconstitution in alkanes solution was supplemented with the 5 esters at high, medium, and low concentration, then analyzed by quantitative descriptive analysis. It was revealed that ethyl octanoate, ethyl tetradecanoate, and Citronellyl acetate adding overall aroma, and geranyl acetate masking the overall aroma perception in a model floral mixture. Sensory profiles highlighted changes in the perception of aroma nuances in the presence of the 5 esters, with specific perceptive interactions, and reported on the graph based on two parameters [sigma=f(tau)]. This paper provided a reference for the flavourists.
Impacts of hormonal elicitors and photoperiod regimes on elicitation of bioactive secondary volatiles in cell cultures of Ajuga bracteosa.[Pubmed:29730585]
J Photochem Photobiol B. 2018 Jun;183:242-250.
Light is an important physical factor necessary for the growth, morphogenesis and production of bioactive compounds in plants. In this study, effects of different photoperiod regimes and hormonal elicitors were investigated on the accumulation of biomass, antioxidant potential and biosynthesis of secondary volatiles in the cell cultures of Ajuga bracteosa. Maximum accumulation of biomass (13.2g/L) was recorded in cell cultures established at 1.0mg/L benzylaminopurine (BA) in exposure to continuous dark. Biochemical assays showed that in the presence of 0.5 methyl jasmonate (Me-J), cell cultures grown under continuous dark had the higher activities of superoxide dismutase (SOD: 4.5U/mg), peroxidase (POD: 3.1U/mg), total phenolic content (TPC: 8.1mg GAE/g of DW) and total flavonoid content (TFC: 5.2mg QE/g of DW) respectively. Nonetheless, the free radical scavenging activity (FRSA) was found correlated with the phenyl ammonia lyase (PAL) activity in the dark grown cell cultures. Analysis through gas chromatography-mass spectrometry (GC-MS) showed, biosynthesis of 29 compounds in the in vitro raised cell cultures. The major identified compounds consisted of monoterpene hydrocarbons such as beta-pinene (2.1-9.5%), beta-ocimene (1.4-8.3%), 1-terpinene-4-ol (5.8-9.6%), caryophyllene (1.3-6.2%), beta-farnesene (0.82-7.8), oxygenated monoterpenes including myrtenal (2.2-8.4%), Citronellyl acetate (2.1-7.3%) and sesquiterpenes such as caryophyllene oxide (1.5-5.5) and beta-elemene (2.2-8.8%). This protocol has the potential for commercial production of important secondary volatiles.
Toxicities of monoterpenes against housefly, Musca domestica L. (Diptera: Muscidae).[Pubmed:28929437]
Environ Sci Pollut Res Int. 2017 Nov;24(31):24708-24713.
The development of natural plant extracts and essential oils will assist to decrease the negative effects of synthetic chemicals. Many plant extracts and essential oils contain monoterpenes, sesquiterpenes, and aliphatic compounds. In the present study, the fumigation activity of 42 pure monoterpenes against housefly, Musca domestica, was evaluated. Results from fumigation tests revealed that rho-cymene, terpinolene, (+/-)-menthol, thymol, carvacrol, (-)-carvone, (+)-camphor, (+)-pulegone, (-)-menthone, citral, (+/-)-citronellal, cuminaldehyde, and Citronellyl acetate exhibited strong fumigation activity against M. domestica. Specifically, the compounds of (+)-pulegone, cuminaldehyde, citral, and rho-cymene had a highest toxicity toward M. domestica with LC50 values of 0.26, 0.60, 0.64, and 0.77 mul/L, respectively. The present results indicated that (+)-pulegone, cuminaldehyde, citral, and rho-cymene are promising toxicants against M. domestica and could be useful in the search for new natural insecticides.
Influence of the chemical structure on the odor characters of beta-citronellol and its oxygenated derivatives.[Pubmed:28490131]
Food Chem. 2017 Oct 1;232:704-711.
beta-Citronellol, 1, and Citronellyl acetate, 2, are renowned fragrant constituents in perfumes and flavoring agents in foods and beverages. Both substances smell citrussy, fresh and floral. To elucidate the structural features required for these sensory effects, six C-8 oxygenated derivatives of 1 and 2 were synthesized and analytically characterized. All compounds were tested for their odor qualities and odor thresholds in air, revealing that there were no significant differences in odor impressions from the parent monoterpenes and their derivatives in most cases; however, substantial differences in their odor threshold values were observed, with beta-citronellol as the most potent (10ng/Lair) and 8-hydroxyCitronellyl acetate as the least potent odorant (1261ng/Lair). 8-OxoCitronellyl acetate was the only compound that was described with divergent odor attributes, namely musty, rotten and coconut-like. 8-Carboxycitronellol and 8-carboxyCitronellyl acetate were found to be odorless.
De novo production of six key grape aroma monoterpenes by a geraniol synthase-engineered S. cerevisiae wine strain.[Pubmed:26377186]
Microb Cell Fact. 2015 Sep 16;14:136.
BACKGROUND: Monoterpenes are important contributors to grape and wine aroma. Moreover, certain monoterpenes have been shown to display health benefits with antimicrobial, anti-inflammatory, anticancer or hypotensive properties amongst others. The aim of this study was to construct self-aromatizing wine yeasts to overproduce de novo these plant metabolites in wines. RESULTS: Expression of the Ocimum basilicum (sweet basil) geraniol synthase (GES) gene in a Saccharomyces cerevisiae wine strain substantially changed the terpene profile of wine produced from a non-aromatic grape variety. Under microvinification conditions, and without compromising other fermentative traits, the recombinant yeast excreted geraniol de novo at an amount (~750 mug/L) well exceeding (>10-fold) its threshold for olfactory perception and also exceeding the quantities present in wines obtained from highly aromatic Muscat grapes. Interestingly, geraniol was further metabolized by yeast enzymes to additional monoterpenes and esters: citronellol, linalool, nerol, Citronellyl acetate and geranyl acetate, resulting in a total monoterpene concentration (~1,558 mug/L) 230-fold greater than that of the control. We also found that monoterpene profiles of wines derived from mixed fermentations were found to be determined by the composition of the initial yeast inocula suggesting the feasibility of producing 'a la carte' wines having predetermined monoterpene contents. CONCLUSIONS: Geraniol synthase-engineered yeasts demonstrate potential in the development of monoterpene enhanced wines.
Application of lipase immobilized on the biocompatible ternary blend polymer matrix for synthesis of citronellyl acetate in non-aqueous media: kinetic modelling study.[Pubmed:24629263]
Enzyme Microb Technol. 2014 Apr 10;57:16-25.
This work reports the use of new support for immobilization of lipase Burkholderia cepacia (BCL) matrix made up of polylactic acid (PLA), chitosan (CH), and polyvinyl alcohol (PVA). Initially lipase from various microbial sources and immobilization support composition was screened to obtain a robust biocatalyst. Among various biocatalysts preparation, the PLA:PVA:CH:BCL (1:6:1:2) was worked as a robust biocatalyst for the Citronellyl acetate synthesis. Various reaction parameters were studied in detail to obtain the suitable reaction conditions for model Citronellyl acetate synthesis reaction. Various kinetic parameters such as r(max), K(i(citronellol)), K(m(citronellol)), K(m(vinyl acetate)) were determined using non-linear regression analysis for the ternary complex as well as bi-bi ping-pong mechanism. The experimental results and kinetic study showed that Citronellyl acetate synthesis catalyzed by immobilized lipase BCL followed the ternary complex mechanism with inhibition by alcohol (citronellol). The energy of activation for Citronellyl acetate synthesis was found to be lower for immobilized lipase (8.9 kcal/mol) than aggregated lipase (14.8 kcal/mol) enzyme. The developed biocatalyst showed four to fivefold higher catalytic activity and excellent recyclability (up to six cycles) than the aggregated lipase.
A new source of elemol rich essential oil and existence of multicellular oil glands in leaves of the Dioscorea species.[Pubmed:24453926]
ScientificWorldJournal. 2013 Dec 23;2013:943598.
Dioscorea species is a very important food and drug plant. The tubers of the plant are extensively used in food and drug purposes owing to the presence of steroidal constituent's diosgenin in the tubers. In the present study, we report for the first time that the leaves of Dioscorea composita and Dioscorea floribunda grown under the field conditions exhibited the presence of multicellular oil glands on the epidermal layers of the plants using stereomicroscopy (SM) and scanning electron microscopy (SEM). Essential oil was also isolated from the otherwise not useful herbage of the plant, and gas chromatographic-mass spectroscopy analysis revealed confirmation of the essential oil constituents. Out of the 76 compounds detected in D. floribunda and 37 from D. composita essential oil, major terpenoids which are detected and reported for Dioscorea leaf essential oil are alpha -terpinene, nerolidol, Citronellyl acetate, farnesol, elemol, alpha -farnesene, valerenyl acetate, and so forth. Elemol was detected as the major constituent of both the Dioscorea species occupying 41% and 22% of D. Floribunda and D. composita essential oils, respectively. In this paper, we report for the first time Dioscorea as a possible novel bioresource for the essential oil besides its well-known importance for yielding diosgenin.
Flowery odor formation revealed by differential expression of monoterpene biosynthetic genes and monoterpene accumulation in rose (Rosa rugosa Thunb.).[Pubmed:24384414]
Plant Physiol Biochem. 2014 Feb;75:80-8.
Rosa rugosa is an important ornamental and economical plant. In this paper, four genes encoding 1-deoxy-D-xylulose-5-phosphate synthase (DXS), 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR), alcohol acyltransferase (AAT) and linalool synthase (LIS) involved in the monoterpene biosynthesis pathways were isolated from R. rugosa 'Tangzi', and the expression patterns of these genes in different flower development stages and different parts of floral organs were determined by real-time quantitative fluorescence PCR. Furthermore, a comprehensive analysis was carried out into the relationship between expression of four monoterpene synthesis genes and accumulation of main volatile monoterpenes and their acetic acid ester derivatives. The results showed that the genes RrDXS, RrDXR and RrLIS showed consistent expressions during the development process for R. rugosa flower from budding to withering stage, the overall expression levels of gene RrDXS and RrLIS were obviously lower as compared with those of gene RrDXR and RrAAT. Although the gene RrDXS, RrDXR, RrAAT and RrLIS were expressed in all parts of R. rugosa floral organs, the expression levels varied significantly. The variations in the constituent and content of volatile monoterpenes including citronellol, geraniol, nerol, linalool, Citronellyl acetate, geranyl acetate and neryl acetate at different development stages and parts of floral organs were significantly different. On this basis, we concluded that the gene RrDXR and RrAAT might play a key role in the biosynthesis of volatile monoterpenes in R. rugosa flowers, and the two genes are important candidate genes for the regulation of secondary metabolism for rose aromatic components.
Bioefficacy of acyclic monoterpenes and their saturated derivatives against the West Nile vector Culex pipiens.[Pubmed:23938144]
Chemosphere. 2014 Feb;96:74-80.
Twenty acyclic monoterpenes with different functional groups (acetoxy, hydroxyl, carbonyl and carboxyl) bearing a variable number of carbon double bonds were assayed as repellent and larvicidal agents against the West Nile vector Culex pipiens. Seven of them were derivatives that were synthesized through either hydrogenation or oxidation procedures. All repellent compounds were tested at the dose of 1mgcm(-2) and only neral and geranial were also tested at a 4-fold lower dose (0.25mgcm(-2)). Repellency results revealed that geranial, neral, nerol, citronellol, geranyl acetate and three more derivatives dihydrolinalool (3), dihydrocitronellol (5) and dihydroCitronellyl acetate (6) resulted in no landings. Based on the LC50 values the derivative dihydroCitronellyl acetate (6) was the most active of all, resulting in an LC50 value of 17.9mgL(-1). Linalyl acetate, Citronellyl acetate, neryl acetate, geranyl acetate, dihydrocitronellol (5), dihydrocitronellal (7), citronellol, dihydrolinalyl acetate (2), citronellic acid and tetrahydrolinalyl acetate (1) were also toxic with LC50 values ranging from 23 to 45mgL(-1). Factors modulating toxicity have been identified, thus providing information on structural requirements for the selected acyclic monoterpenes. The acetoxy group enhanced toxicity, without being significantly affected by the unsaturation degree. Within esters, reduction of the vinyl group appears to decrease potency. Presence of a hydroxyl or carbonyl group resulted in increased activity but only in correlation to saturation degree. Branched alcohols proved ineffective compared to the corresponding linear isomers. Finally, as it concerns acids, data do not allow generalizations or correlations to be made.


