10(14)-Cadinene-4,5-diolCAS# 672336-50-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 672336-50-4 | SDF | Download SDF |
| PubChem ID | 11053721 | Appearance | Oil |
| Formula | C15H26O2 | M.Wt | 238.4 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
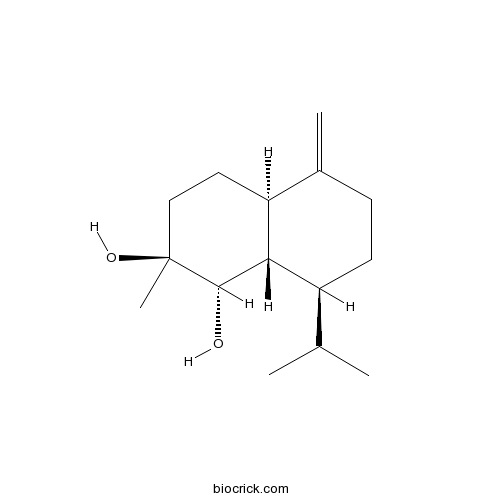
| Chemical Name | (1S,2S,4aR,8S,8aS)-2-methyl-5-methylidene-8-propan-2-yl-1,3,4,4a,6,7,8,8a-octahydronaphthalene-1,2-diol | ||
| SMILES | CC(C)C1CCC(=C)C2C1C(C(CC2)(C)O)O | ||
| Standard InChIKey | DQVXKPUFGSPUGZ-YTFOTSKYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

10(14)-Cadinene-4,5-diol Dilution Calculator

10(14)-Cadinene-4,5-diol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1946 mL | 20.9732 mL | 41.9463 mL | 83.8926 mL | 104.8658 mL |
| 5 mM | 0.8389 mL | 4.1946 mL | 8.3893 mL | 16.7785 mL | 20.9732 mL |
| 10 mM | 0.4195 mL | 2.0973 mL | 4.1946 mL | 8.3893 mL | 10.4866 mL |
| 50 mM | 0.0839 mL | 0.4195 mL | 0.8389 mL | 1.6779 mL | 2.0973 mL |
| 100 mM | 0.0419 mL | 0.2097 mL | 0.4195 mL | 0.8389 mL | 1.0487 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fenoldopam
Catalog No.:BCC4462
CAS No.:67227-56-9
- 5'-O-Dimethoxytrityl-N-benzoyl-desoxycytidine
Catalog No.:BCC8750
CAS No.:67219-55-0
- 2'-O-Coumaroyljuglanin
Catalog No.:BCN6559
CAS No.:67214-05-5
- SMER 3
Catalog No.:BCC6152
CAS No.:67200-34-4
- H-HoSer-OH
Catalog No.:BCC3247
CAS No.:672-15-1
- (±)-U-50488 hydrochloride
Catalog No.:BCC6665
CAS No.:67197-96-0
- Moronic acid
Catalog No.:BCN4222
CAS No.:6713-27-5
- Yamataimine
Catalog No.:BCN2064
CAS No.:67113-69-3
- Tubotaiwine
Catalog No.:BCN4016
CAS No.:6711-69-9
- Cyclo(Pro-Pro)
Catalog No.:BCN2415
CAS No.:6708-06-1
- Datumetine
Catalog No.:BCN1930
CAS No.:67078-20-0
- Trichophydine
Catalog No.:BCN1781
CAS No.:67031-54-3
- Boc-MLF
Catalog No.:BCC6061
CAS No.:67247-12-5
- Cabraleadiol
Catalog No.:BCN4224
CAS No.:67253-01-4
- SKF 81297 hydrobromide
Catalog No.:BCC7071
CAS No.:67287-39-2
- SKF 83959 hydrobromide
Catalog No.:BCC7251
CAS No.:67287-95-0
-
γDGG
Catalog No.:BCC6557
CAS No.:6729-55-1
- Kif15-IN-1
Catalog No.:BCC5152
CAS No.:672926-32-8
- Kif15-IN-2
Catalog No.:BCC5153
CAS No.:672926-33-9
- 3-O-(2'E ,4'Z-decadienoyl)-20-deoxyingenol
Catalog No.:BCN1382
CAS No.:672941-64-9
- Kansuinine E
Catalog No.:BCN3769
CAS No.:672945-84-5
- H-D-Phe-OH
Catalog No.:BCC3012
CAS No.:673-06-3
- H-Asp(OtBu)-OMe.HCl
Catalog No.:BCC2892
CAS No.:2673-19-0
- 2-Hydroxy-4-methoxybenzaldehyde
Catalog No.:BCN7798
CAS No.:673-22-3
Phytotoxic activity of bibenzyl derivatives from the orchid Epidendrum rigidum.[Pubmed:16076106]
J Agric Food Chem. 2005 Aug 10;53(16):6276-80.
A whole plant chloroform-methanol extract of the orchid Epidendrum rigidum inhibited radicle growth of Amaranthus hypochondriacus seedlings (IC50 = 300 microg/mL). Bioassay-guided fractionation furnished four phytotoxins, namely, gigantol (1), batatasin III (2), 2,3-dimethoxy-9,10-dihydrophenathrene-4,7-diol (9), and 3,4,9-trimethoxyphenanthrene-2,5-diol (11), along with the known flavonoids apigenin, vitexin, and isovetin and the triterterpenoids 24,24-dimethyl-9,19-cyclolanostane-25-en-3beta-ol (14) and 24-methyl-9,19-cyclolanostane-25-en-3beta-ol (15). Stilbenoids 1, 2, 9, and 11 inhibited radicle growth of A. hypochondriacus with IC50 values of 0.65, 0.1, 0.12, and 5.9 microM, respectively. Foliar application of gigantol (1) at 1 microM to 4 week old seedlings of A. hypochondriacus reduced shoot elongation by 69% and fresh weight accumulation by 54%. Bibenzyls 1 and 2, as well as synthetic analogues 4'-hydroxy-3,3',5-trimethoxybibenzyl (3), 3,3',4',5-tetramethoxybibenzyl (4), 3,4'-dihydroxy-5-methoxybibenzyl (5), 3'-O-methylbatatasin III (6), 3,3',5-trihydroxybibenzyl (7), and 3,4',5-trihydroxybibenzyl (8), were tested for phytotoxicity in axenic cultures of the small aquatic plant Lemna pausicostata. All bibenzyls derivatives except 7 and 8 inhibited growth and increased cellular leakage with IC50 values of 89.9-180 and 89.9-166 microM, respectively. The natural and synthetic bibenzyls showed marginal cytotoxicity on animal cells. The results suggest that orchid bibenzyls may be good lead compounds for the development of novel herbicidal agents.
Muurolane-type sesquiterpenes from marine sponge Dysidea cinerea.[Pubmed:24243694]
Magn Reson Chem. 2014 Jan-Feb;52(1-2):51-6.
Seven new muurolane-type sesquiterpenes, (4R,5R)-muurol-1(6),10(14)-diene-4,5-diol (1), (4R,5R)-muurol-1(6)-ene-4,5-diol (2), (4R,5R,10R)-10-methoxymuurol-1(6)-ene-4,5-diol (3), (4S)-4-hydroxy-1,10-seco-muurol-5-ene-1,10-dione (4), (4R)-4-hydroxy-1,10-seco-muurol-5-ene-1,10-dione (5), (6S,10S)-6,10-dihydroxy-7,8-seco-2,8-cyclo-muurol-4(5),7(11)-diene-12-oic acid (6), and (6R,10S)-6,10-dihydroxy-7,8-seco-2,8-cyclo-muurol-4(5),7(11)-diene-12-oic acid (7) were isolated from the marine sponge Dysidea cinerea. Their structures were determined by the combination of spectroscopic and chemical methods, including 1D-NMR, 2D-NMR, and CD spectra as well as by comparing the NMR data with those reported in the literature.
Bioactive Constituents from the Whole Plants of Gentianella acuta (Michx.) Hulten.[Pubmed:28783086]
Molecules. 2017 Aug 6;22(8). pii: molecules22081309.
As a Mongolian native medicine and Ewenki folk medicinal plant, Gentianella acuta has been widely used for the treatment of diarrhea, hepatitis, arrhythmia, and coronary heart disease. In the course of investigating efficacy compounds to treat diarrhea using a mouse isolated intestine tissue model, we found 70% EtOH extract of G. acuta whole plants had an inhibitory effect on intestine contraction tension. Here, nineteen constituents, including five new compounds, named as gentiiridosides A (1), B (2), gentilignanoside A (3), (1R)-2,2,3-trimethyl-4-hydroxymethylcyclopent-3-ene-1-methyl-O-beta-d-glucopyrano side (4), and (3Z)-3-hexene-1,5-diol 1-O-alpha-l-arabinopyranosyl(1-->6)-beta-d-glucopyranoside (5) were obtained from it. The structures of them were elucidated by chemical and spectroscopic methods. Furthermore, the inhibitory effects on motility of mouse isolated intestine tissue of the above mentioned compounds and other thirteen iridoid- and secoiridoid-type monoterpenes (7-10, 13-16, 18, 19, 21, 22, and 25) previously obtained in the plant were analyzed. As results, new compound 5, some secoiridoid-type monoterpenes 7, 10, 12-14, 16, and 17, as well as 7-O-9'-type lignans 31 and 32 displayed significant inhibitory effect on contraction tension at 40 muM.
Toxicity of propargylic alcohols on green alga--Pseudokirchneriella subcapitata.[Pubmed:22105539]
J Environ Monit. 2012 Jan;14(1):181-6.
The present study evaluates the toxicity of 34 propargylic alcohols, including primary, primary homo-, secondary, and tertiary alcohols, based on their effects on phytoplankton. A closed-system algal toxicity test was applied because the closed-system technique presents more realistic concentration-response relationships for the above compounds than the conventional batch tests. The green alga, Pseudokirchneriella subcapitata, was the test organism and final yield and growth rate were chosen as the test endpoints. Among all the propargylic alcohols tested, 1-pentyn-3-ol is the most toxic compound with its EC50 equal to 0.50 mg L(-1), which can be classified as a "R50" compound (very toxic to aquatic organisms, EC50/LC50 < 1 mg L(-1)), following the current practice for classification of chemicals in the European Union (EU). There are several other compounds including 2-decyn-1-ol, 3-decyn-1-ol, 1-hexyn-3-ol, 3-butyn-2-ol, and 3-hexyne-2,5-diol, which deserve more attention for their possible adverse impact on the aquatic environment, because these alcohols can be classified as "R51" compounds (toxic to aquatic organisms, EC50/LC50 between 1 and 10 mg L(-1)). Compared to the base-line toxicity relationship (narcosis QSAR) derived previously, tertiary propargylic alcohols can be identified as nonpolar narcotic chemicals, while secondary alcohols and primary alcohols with low molecular weight generally exhibit obvious excess toxicity in relation to the base-line toxicity. Finally, quantitative structure-activity relationships were established for deriving a preliminary estimation of the toxicity of other propargylic alcohols.
C(1)(4)-polyacetylene glucosides from Codonopsis pilosula.[Pubmed:26009940]
J Asian Nat Prod Res. 2015;17(6):601-14.
Seven new C14-polyacetylene glucosides codonopilodiynosides A-G (1-7) were isolated from an aqueous extract of the Codonopsis pilosula roots. Their structures were determined by spectroscopic and chemical methods as (-)-(5S,6E,12E)-tetradeca-6,12-dien-8,10-diyn-1,5,14-triol 5-O-beta-D-glucopyranoside (1), (-)-(5S,6E,12E)-tetradeca-6,12-dien-8,10-diyn-1,5,14-triol 5-O-beta-D-glucopyranosyl-(1'' --> 2')-beta-D-glucopyranoside (2), (-)-(5S,6E,12E)-tetradeca-6,12-dien-8,10-diyn-1,5,14-triol 5,14-di-O-beta-D-glucopyranoside (3), (-)-(5S,6E)-tetradeca-6-en-8,10-diyn-1,5,14-triol 5-O-beta-D-glucopyranoside (4), (-)-(5S,6E,12E)-tetradeca-6,12-dien-8,10-diyn-1,5-diol 5-O-beta-D-glucopyranosyl-(1'' --> 2')-beta-D-glucopyranoside (5), (-)-(6S,4E,12E)-tetradeca-4,12-dien-8,10-diyn-1,6-diol 6-O-beta-D-glucopyranosyl-(1'' --> 2')-beta-D-glucopyranoside (6), and (-)-(5S,6E)-tetradeca-6-en-1,5-epoxy-8,10-diyn-14-ol 14-O-beta-D-glucopyranosyl-(1'' --> 2')-beta-D-glucopyranoside (7), respectively. The absolute configurations of 1-7 were assigned by enzymatic hydrolysis followed by isolation of glucose and aglycones (1a and 4a-7a), and subsequent comparison of specific rotation, TLC, and (1)H NMR data of the glucose with an authentic sugar sample and application of modified Mosher's method based on the MPA determination rule of Deltadelta(RS) values for 1a and 4a, and Deltadelta(S) values for 6a. The configuration of 7 was assigned by electronic circular dichroism calculations based on the quantum-mechanical time-dependent density functional theory.


