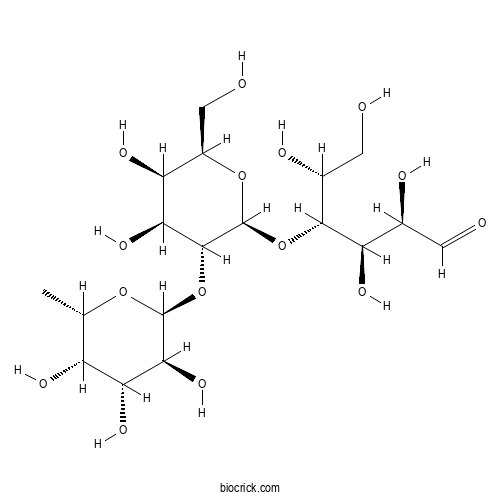
2'-FucosyllactoseCAS# 41263-94-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 41263-94-9 | SDF | Download SDF |
| PubChem ID | 170484.0 | Appearance | Powder |
| Formula | C18H32O15 | M.Wt | 488.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2R,3R,4R,5R)-4-[(2S,3R,4S,5R,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-[(2S,3S,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl]oxy-2,3,5,6-tetrahydroxyhexanal | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(C(OC2OC(C(CO)O)C(C(C=O)O)O)CO)O)O)O)O)O | ||
| Standard InChIKey | HWHQUWQCBPAQQH-BWRPKUOHSA-N | ||
| Standard InChI | InChI=1S/C18H32O15/c1-5-9(24)12(27)14(29)17(30-5)33-16-13(28)11(26)8(4-21)31-18(16)32-15(7(23)3-20)10(25)6(22)2-19/h2,5-18,20-29H,3-4H2,1H3/t5-,6-,7+,8+,9+,10+,11-,12+,13-,14-,15+,16+,17-,18-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

2'-Fucosyllactose Dilution Calculator

2'-Fucosyllactose Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0473 mL | 10.2367 mL | 20.4733 mL | 40.9467 mL | 51.1834 mL |
| 5 mM | 0.4095 mL | 2.0473 mL | 4.0947 mL | 8.1893 mL | 10.2367 mL |
| 10 mM | 0.2047 mL | 1.0237 mL | 2.0473 mL | 4.0947 mL | 5.1183 mL |
| 50 mM | 0.0409 mL | 0.2047 mL | 0.4095 mL | 0.8189 mL | 1.0237 mL |
| 100 mM | 0.0205 mL | 0.1024 mL | 0.2047 mL | 0.4095 mL | 0.5118 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Myriacetin
Catalog No.:BCX1059
CAS No.:203734-35-4
- Phloretin 2'-xyloglucoside
Catalog No.:BCX1058
CAS No.:145758-09-4
- 5-Hydroxyferulic acid
Catalog No.:BCX1057
CAS No.:1782-55-4
- 3-Fucosyllactose
Catalog No.:BCX1056
CAS No.:41312-47-4
- Isohanalpinone
Catalog No.:BCX1055
CAS No.:103476-95-5
- Paeonidanin
Catalog No.:BCX1054
CAS No.:209969-75-5
- Isotheaflavin
Catalog No.:BCX1053
CAS No.:31701-93-6
- 11-Methoxyyangonin
Catalog No.:BCX1052
CAS No.:2743-14-8
- Phenaxolactone 1
Catalog No.:BCX1051
CAS No.:147022-96-6
- 3'-O-Acetylhamaudol
Catalog No.:BCX1050
CAS No.:30358-88-4
- Pterostilbene glucoside
Catalog No.:BCX1049
CAS No.:38967-99-6
- 2"-Acetylhyperin
Catalog No.:BCX1048
CAS No.:439266-62-3
- Paeoniflorin sulfite
Catalog No.:BCX1061
CAS No.:1146967-98-7
- (+)-Epitaxifolin
Catalog No.:BCX1062
CAS No.:153666-25-2
- Notoginsenoside ST4
Catalog No.:BCX1063
CAS No.:155683-02-6
- Podecdysone B
Catalog No.:BCX1064
CAS No.:22612-27-7
- Crocetine dimethyl ester
Catalog No.:BCX1065
CAS No.:5892-54-6
- Resveratrol 12-C-β-glucopyranoside
Catalog No.:BCX1066
CAS No.:163527-00-2
- Cochinchinenin A
Catalog No.:BCX1067
CAS No.:1057666-04-2
- trans-p-Coumaric acid 4-O-β-D-glucopyranoside
Catalog No.:BCX1068
CAS No.:117405-49-9
- Tamarixetin-3-O-rutinoside
Catalog No.:BCX1069
CAS No.:20550-05-4
- 2-O-α-D-Glucopyranosyl-L-ascorbic acid
Catalog No.:BCX1070
CAS No.:129499-78-1
- Stachyanthuside A
Catalog No.:BCX1071
CAS No.:864779-30-6
- Prunetinoside
Catalog No.:BCX1072
CAS No.:89595-66-4
Innovative direct introduction-ion mobility-mass spectrometry (DI-IM-MS) approach for fast and robust isomer-specific quantification in a complex matrix: Application to 2'-fucosyllactose (2'-FL) in breast milk.[Pubmed:38656572]
J Mass Spectrom. 2024 May;59(5):e5026.
Identification and specific quantification of isomers in a complex biological matrix by mass spectrometry alone is not an easy task due to their identical chemical formula and therefore their same mass-to-charge ratio (m/z). Here, the potential of direct introduction combined with ion mobility-mass spectrometry (DI-IM-MS) for rapid quantification of isomers as human milk oligosaccharides (HMOs) was investigated. Differences in HMO profiles between various analyzed breast milk samples were highlighted using the single ion mobility monitoring (SIM(2)) acquisition for high ion mobility resolution detection. Furthermore, the Se+ (secretor) or Se- (non-secretor) phenotype could be assigned to breast milk samples studied based on their HMO contents, especially on the response of 2'-fucosyllactose (2'-FL) and lacto-N-fucopentaose I (LNFP I). The possibility of quantifying a specific isomer in breast milk by DI-IM-MS was also investigated. The standard addition method allowed the determination of the 2'-FL despite the presence of other oligosaccharides, including 3-fucosyllactose (3-FL) isomer in breast milk. This proof-of-concept study demonstrated the high potential of such an approach for the rapid and convenient quantification of isomers in complex mixtures.
Validation of collection and anaerobic fermentation techniques for measuring prebiotic impact on gut microbiota.[Pubmed:38583688]
Pharmacol Res. 2024 May;203:107169.
BACKGROUND: Defining the ability of prebiotic dietary carbohydrates to influence the composition and metabolism of the gut microbiota is central to defining their health impact in diverse individuals. Many clinical trials are using indirect methods. This study aimed to validate collection and fermentation methods enabling their use in the context of clinical studies. METHODS AND RESULTS: Parameters tested included stool sample acquisition, storage, and growth conditions. Stool from 3 infants and 3 adults was collected and stored under varying conditions. Samples were cultured anaerobically for two days in the presence of prebiotics, whereupon optical density and pH were measured across time. Whole genome shotgun sequencing and NMR metabolomics were performed. Neither the type of collection vial (standard vial and two different BD anaerobic collection vials) nor cryopreservation (-80 degrees C or 4 degrees C) significantly influenced either microbial composition at 16 h of anaerobic culture or the principal components of the metabolome at 8 or 16 h. Metagenomic differences were driven primarily by subject, while metabolomic differences were driven by fermentation sugar (2'-fucosyllactose or dextrose). CONCLUSIONS: These data identified a feasible and valid approach for prebiotic fermentation analysis of individual samples in large clinical studies: collection of stool microbiota using standard vials; cryopreservation prior to testing; and collecting fermentation read-out at 8 and 16 hr. Thus, fermentation analysis can be a valid technique for testing the effects of prebiotics on human fecal microbiota.
Effects of 2'-fucosyllactose on the viability of starter cultures and Bifidobacterium strains of human origin in yogurt during refrigerated storage.[Pubmed:38578148]
J Food Sci. 2024 Apr 5.
2'-Fucosyllactose (2'-FL) is postulated to provide health benefits and promote the growth of probiotics. This work was undertaken to study the effects of 2'-FL on the viability of starter cultures and Bifidobacterium strains of human origin in yogurt during refrigerated storage. Yogurts were produced containing 2'-FL (0 or 2 g/L) and Bifidobacterium strains of human origin (Bifidobacterium longum subsp. longum BB536 or Bifidobacterium longum subsp. infantis ATCC 15697) at a concentration of at least 10(9) CFU/mL. All yogurts were stored at 4 degrees C for 5 weeks. Results showed that 2'-FL was stable in yogurts for at least 5 weeks of cold storage, and the addition of 2'-FL did not significantly alter yogurt fermentation parameters, associated metabolites, and the viability of mixed yogurt starter cultures and Bifidobacterium strains (p > 0.05). The addition of bifidobacteria had a negative impact (p < 0.05) on the survival rate of starter cultures, Streptococcus thermophilus and Lactobacillus delbureckii subsp. bulgaricus. Meanwhile, it is difficult to maintain a high survival rate of bifidobacteria in final yogurt products, and the addition of 2'-FL could not enhance the viability of bifidobacteria. B. longum BB536 survived at a level higher than 10(6) CFU/g for 28 days, while B. infantis ATCC15697 maintained this level for only 7 days. In summary, this study has shown the impact of 2'-FL and bifidobacterial species on yogurt properties, and results suggest that it is promising to use 2'-FL in yogurt products as a prebiotic. PRACTICAL APPLICATION: Yogurt is known for its beneficial effects on human health and nutrition. This study reported the production of symbiotic yogurt containing bifidobacteria and 2'-fucosyllactose (2'-FL) as a functional food for specified health uses. The viability of yogurt starter cultures and probiotic bifidobacterial strains was analyzed in this study. Moreover, this research demonstrated that 2'-FL could be added to yogurt without affecting the characteristics of yogurt significantly.
Enhancing the soluble expression of alpha-1,2-fucosyltransferase in E. coli using high-throughput flow cytometry screening coupled with a split-GFP.[Pubmed:38556215]
J Biotechnol. 2024 May 20;387:49-57.
2'-Fucosyllactose (2'-FL), one of the major human milk oligosaccharides, was produced in several engineered microorganisms. However, the low solubility of alpha-1,2-fucosyltransferase (alpha1,2-FucT) often becomes a bottleneck to produce maximum amount of 2'-FL in the microorganisms. To overcome this solubility issue, the following studies were conducted to improve the soluble expression of alpha1,2-FucT. Initially, hydrophobic amino acids in the hydrophilic region of the 6 alpha-helices were mutated, adhering to the alpha-helix rule. Subsequently, gfp11 was fused to the C-terminal of futC gene encoding alpha1,2-FucT (FutC), enabling selection of high-fluorescence mutants through split-GFP. Each mutant library was screened via fluorescence activated cell sorting (FACS) to separate soluble mutants for high-throughput screening. As a result, L80C single mutant and A121D/P124A/L125R triple mutant were found, and a combined quadruple mutant was created. Furthermore, we combined mutations of conserved sequences (Q150H/C151R/Q239S) of FutC, which showed positive effects in the previous studies from our lab, with the above quadruple mutants (L80C/A121D/P124A/L125R). The resulting strain produced approximately 3.4-fold higher 2'-FL titer than that of the wild-type, suggesting that the conserved sequence mutations are an independent subset of the mutations that further improve the solubility of the target protein acquired by random mutagenesis using split-GFP.
An In Vitro Colonic Fermentation Study of the Effects of Human Milk Oligosaccharides on Gut Microbiota and Short-Chain Fatty Acid Production in Infants Aged 0-6 Months.[Pubmed:38540911]
Foods. 2024 Mar 18;13(6):921.
The impact of five human milk oligosaccharides (HMOs)-2'-fucosyllactose (2FL), 3'-sialyllactose (3SL), 6'-sialyllactose (6SL), lacto-N-tetraose (LNT), and lacto-N-neotetraose (LNnT)-on the gut microbiota and short-chain fatty acid (SCFA) metabolites in infants aged 0-6 months was assessed through in vitro fermentation. Analyses of the influence of different HMOs on the composition and distribution of infant gut microbiota and on SCFA levels were conducted using 16S rRNA sequencing, quantitative real-time PCR (qPCR), and gas chromatography (GC), respectively. The findings indicated the crucial role of the initial microbiota composition in shaping fermentation outcomes. Fermentation maintained the dominant genera species in the intestine but influenced their abundance and distribution. Most of the 10 Bifidobacteria strains effectively utilized HMOs or their degradation products, particularly demonstrating proficiency in utilizing 2FL and sialylated HMOs compared to non-fucosylated neutral HMOs. Moreover, our study using B. infantis-dominant strains and B. breve-dominant strains as inocula revealed varying acetic acid levels produced by Bifidobacteria upon HMO degradation. Specifically, the B. infantis-dominant strain yielded notably higher acetic acid levels than the B. breve-dominant strain (p = 0.000), with minimal propionic and butyric acid production observed at fermentation's conclusion. These findings suggest the potential utilization of HMOs in developing microbiota-targeted foods for infants.
Air pollution exposure may impact the composition of human milk oligosaccharides.[Pubmed:38509153]
Sci Rep. 2024 Mar 20;14(1):6730.
Human milk oligosaccharides (HMOs) impact neonate immunity and health outcomes. However, the environmental factors influencing HMO composition remain understudied. This study examined the associations between ambient air pollutant (AAP) exposure and HMOs at 1-month postpartum. Human milk samples were collected at 1-month postpartum (n = 185). AAP (PM(2.5), PM(10), NO(2)) exposure included the 9-month pregnancy period through 1-month postpartum. Associations between AAP with (1) HMO diversity, (2) the sum of sialylated and fucosylated HMOs, (3) 6 a priori HMOs linked with infant health, and (4) all HMOs were examined using multivariable linear regression and principal component analysis (PCA). Exposure to AAP was associated with lower HMO diversity. PM(2.5) and PM(10) exposure was positively associated with the HMO 3-fucosyllactose (3FL); PM(2.5) exposure was positively associated with the sum of total HMOs, sum of fucosylated HMOs, and the HMO 2'-fucosyllactose (2'FL). PCA indicated the PM(2.5), PM(10), and NO(2) exposures were associated with HMO profiles. Individual models indicated that AAP exposure was associated with five additional HMOs (LNFP I, LNFP II, DFLNT, LNH). This is the first study to demonstrate associations between AAP and breast milk HMOs. Future longitudinal studies will help determine the long-term impact of AAP on human milk composition.
Ameliorating effect of 2'-Fucosyllactose and 6'-Sialyllactose on lipopolysaccharide-induced intestinal inflammation.[Pubmed:38490539]
J Dairy Sci. 2024 Mar 13:S0022-0302(24)00568-X.
Human milk oligosaccharides (HMO) affect gut microbiota during neonatal development, particularly with respect to the immune system. Bovine milk-based infant formulas have low oligosaccharide contents. Thus, efforts to fortify infant formulas with HMO are being undertaken. Two major HMO, 2'-fucosyllactose (2'-FL) and 6'-sialyllactose (6'-SL), exert anti-inflammatory effects; however, the associations between anti-inflammatory effects induced by 2'-FL and 6'-SL co-treatment and gut microbiota composition and metabolite modulation remain unclear. Therefore, in this study, we evaluated the effects of a mixture of these HMO. To determine the optimal HMO ratio for anti-inflammatory effects and elucidate its mode of action, LPS-induced inflammatory HT-29 epithelial cells and intestinal inflamed suckling mice were treated with various mixtures of 2'-FL and 6'-SL. 2'-FL:6'-SL ratio of 5:1 was identified as the most effective pre-treatment HMO mixture in vitro; thus, this ratio was selected and used for low, middle, and high-dose treatments for subsequent in vivo studies. In vivo, high-dose HMO treatment restored LPS-induced inflammation symptoms, such as body weight loss, colon length reduction, histological structural damage, and intestinal gene expression related to inflammatory responses. High-dose HMO was the only treatment that modulated the major phyla Bacteroidetes and Firmicutes and the genera Ihubacter, Mageeibacillus, and Saccharofermentans. These changes in microbial composition were correlated with intestinal inflammation-related gene expression and short-chain fatty acid production. To our knowledge, our study is the first to report the effects of Ihubacter, Mageeibacillus, and Saccharofermentans on short chain fatty acid levels, which can subsequently affect inflammatory cytokine and tight junction protein levels. Conclusively, the HMO mixture exerted anti-inflammatory effects through changes in microbiota and metabolite production. These findings suggested that supplementation of infant formula with HMO may benefit formula-fed infants by forming unique microbiota contributing to neonatal development.
Screening competition and cross-feeding interactions during utilization of human milk oligosaccharides by gut microbes.[Pubmed:38455082]
Microbiome Res Rep. 2024 Jan 1;3(1):12.
Background: The infant gut microbiome is a complex community that influences short- and long-term health. Its assembly and composition are governed by variables such as the feeding type. Breast milk provides infants an important supply of human milk oligosaccharides (HMO), a broad family of carbohydrates comprising neutral, fucosylated, and sialylated molecules. There is a positive association between HMOs and the overrepresentation of Bifidobacterium species in the infant gut, which is sustained by multiple molecular determinants present in the genomes of these species. Infant-gut-associated Bifidobacterium species usually share a similar niche and display similar HMO inclinations, suggesting they compete for these resources. There is also strong evidence of cross-feeding interactions between HMO-derived molecules and bifidobacteria. Methods: In this study, we screened for unidirectional and bidirectional interactions between Bifidobacterium and other species using individual HMO. Bifidobacterium bifidum and Bacteroides thetaiotaomicron increased the growth of several other species when their supernatants were used, probably mediated by the partial degradation of HMO. In contrast, Bifidobacterium longum subsp. infantis. supernatants did not exhibit positive growth. Results: Bifidobacterium species compete for lacto-N-tetraose, which is associated with reduced bidirectional growth. The outcome of these interactions was HMO-dependent, in which the two species could compete for one substrate but cross-feed on another. 2'-fucosyllactose and lacto-N-neotetraose are associated with several positive interactions that generally originate from the partial degradation of these HMOs. Conclusion: This study presents evidence for complex interactions during HMO utilization, which can be cooperative or competitive, depending on the nature of the HMO. This information could be useful for understanding how breast milk supports the growth of some Bifidobacterium species, shaping the ecology of this important microbial community.
A human milk oligosaccharide prevents intestinal inflammation in adulthood via modulating gut microbial metabolism.[Pubmed:38441000]
mBio. 2024 Apr 10;15(4):e0029824.
Observational evidence suggests that human milk oligosaccharides (HMOs) promote the growth of commensal bacteria in early life and adulthood. However, the mechanisms by which HMOs benefit health through modulation of gut microbial homeostasis remain largely unknown. 2'-fucosyllactose (2'-FL) is the most abundant oligosaccharide in human milk and contributes to the essential health benefits associated with human milk consumption. Here, we investigated how 2'-FL prevents colitis in adulthood through its effects on the gut microbial community. We found that the gut microbiota from adult mice that consumed 2'-FL exhibited an increase in abundance of several health-associated genera, including Bifidobacterium and Lactobacillus. The 2'-FL-modulated gut microbial community exerted preventive effects on colitis in adult mice. By using Bifidobacterium infantis as a 2'-FL-consuming bacterial model, exploratory metabolomics revealed novel 2'-FL-enriched secretory metabolites by Bifidobacterium infantis, including pantothenol. Importantly, pantothenate significantly protected the intestinal barrier against oxidative stress and mitigated colitis in adult mice. Furthermore, microbial metabolic pathway analysis identified 26 dysregulated metabolic pathways in fecal microbiota from patients with ulcerative colitis, which were significantly regulated by 2'-FL treatment in adult mice, indicating that 2'-FL has the potential to rectify dysregulated microbial metabolism in colitis. These findings support the contribution of the 2'-FL-shaped gut microbial community and bacterial metabolite production to the protection of intestinal integrity and prevention of intestinal inflammation in adulthood.IMPORTANCEAt present, neither basic research nor clinical studies have revealed the exact biological functions or mechanisms of action of individual oligosaccharides during development or in adulthood. Thus, it remains largely unknown whether human milk oligosaccharides could serve as effective therapeutics for gastrointestinal-related diseases. Results from the present study uncover 2'-FL-driven alterations in bacterial metabolism and identify novel B. infantis-secreted metabolites following the consumption of 2'-FL, including pantothenol. This work further demonstrates a previously unrecognized role of pantothenate in significantly protecting the intestinal barrier against oxidative stress and mitigating colitis in adult mice. Remarkably, 2'-FL-enhanced bacterial metabolic pathways are found to be dysregulated in the fecal microbiota of ulcerative colitis patients. These novel metabolic pathways underlying the bioactivities of 2'-FL may lay a foundation for applying individual oligosaccharides for prophylactic intervention for diseases associated with impaired intestinal homeostasis.
2'-Fucosyllactose Promotes Colonization of Akkermansia muciniphila and Prevents Colitis In Vitro and in Mice.[Pubmed:38393978]
J Agric Food Chem. 2024 Mar 6;72(9):4765-4776.
Akkermansia muciniphila is a potential candidate for ulcerative colitis prevention. Considering that it utilizes 2'-fucosyllactose (2'FL) for growth, 2'FL can be used to enrich the abundance of A. muciniphila in feces. However, whether the crosswalk between 2'FL and A. muciniphila can promote the intestinal colonization of A. muciniphila remains unclear. In this study, we explored the effect and the underlying mechanism of 2'FL on the colonization of A. muciniphila in vitro and in vivo as well as its alleviating effect on colitis. Our results revealed that 2'FL can serve as a carbon source of A. muciniphila to support the growth and increase cell-surface hydrophobicity and the expression of the genes coding fibronectin-binding autotransporter adhesin to promote the adhesion to Caco2/HT29 methotrexate (MTX) cells but not of galactooligosaccharides (GOS) and glucose. Moreover, 2'FL could increase the host mucin formation to promote the adhesion of A. muciniphila to Caco2/HT29 MTX cells but not of GOS and glucose. Furthermore, 2'FL could significantly increase the colonization of A. muciniphila in the gut to alleviate colitis in mice. Overall, the interplay between A. muciniphila and 2'FL is expected to provide an advantageous ecological niche for A. muciniphila so as to confer further health benefits against colitis.
2'-Fucosyllactose helps butyrate producers outgrow competitors in infant gut microbiota simulations.[Pubmed:38380251]
iScience. 2024 Feb 3;27(3):109085.
A reduced capacity for butyrate production by the early infant gut microbiota is associated with negative health effects, such as inflammation and the development of allergies. Here, we develop new hypotheses on the effect of the prebiotic galacto-oligosaccharides (GOS) or 2'-fucosyllactose (2'-FL) on butyrate production by the infant gut microbiota using a multiscale, spatiotemporal mathematical model of the infant gut. The model simulates a community of cross-feeding gut bacteria in metabolic detail. It represents the community as a grid of bacterial populations that exchange metabolites, using 20 different subspecies-specific metabolic networks taken from the AGORA database. The simulations predict that both GOS and 2'-FL promote the growth of Bifidobacterium, whereas butyrate producing bacteria are only consistently abundant in the presence of propane-1,2-diol, a product of 2'-FL metabolism. In absence of prebiotics or in presence of only GOS, however, Bacteroides vulgatus and Cutibacterium acnes outcompete butyrate producers by consuming intermediate metabolites.


