Notoginsenoside ST4CAS# 155683-02-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 155683-02-6 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
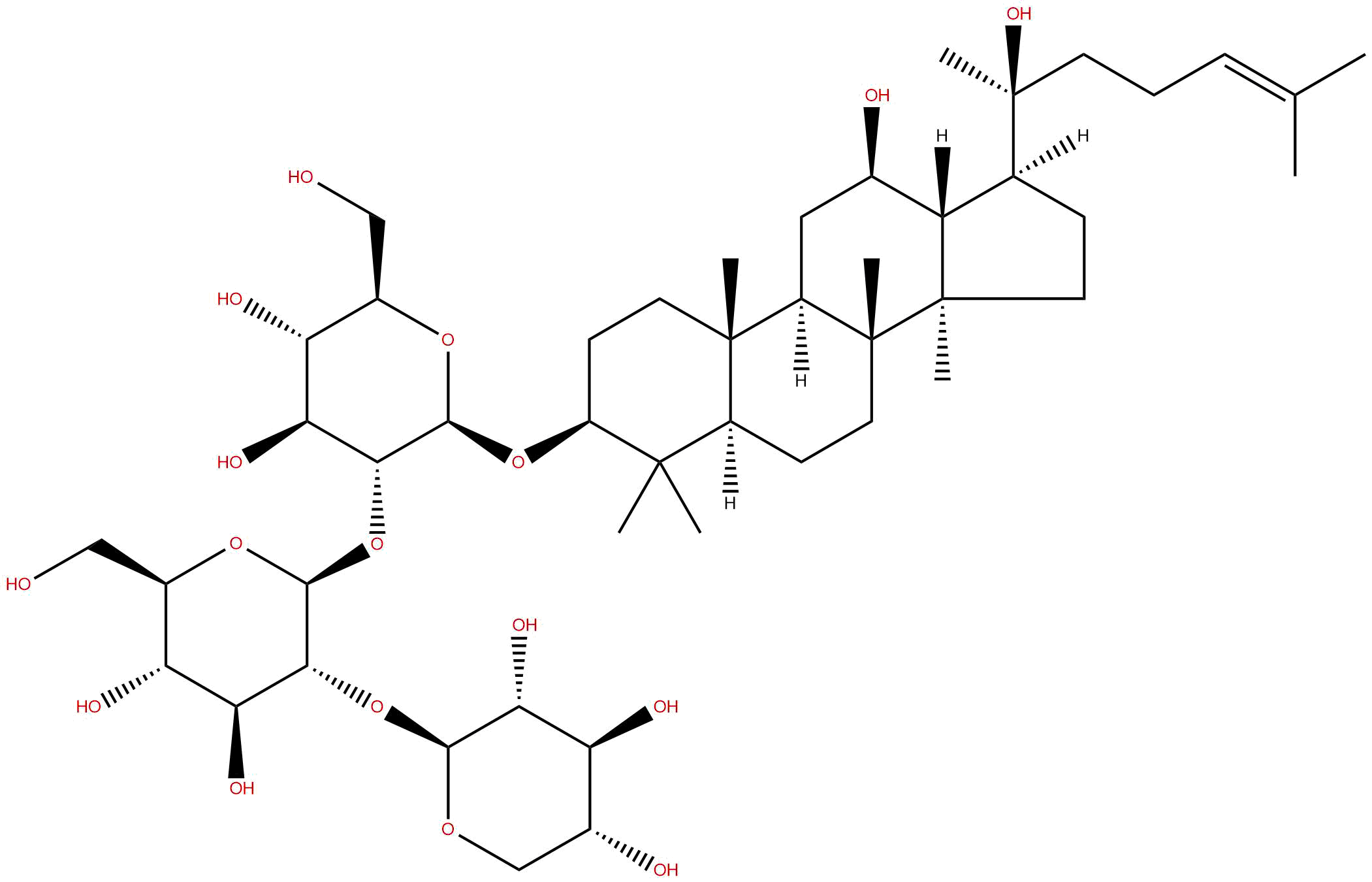
| Formula | C47H80O17 | M.Wt | 917.14 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Notoginsenoside ST-4 | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Notoginsenoside ST4 Dilution Calculator

Notoginsenoside ST4 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0903 mL | 5.4517 mL | 10.9035 mL | 21.8069 mL | 27.2587 mL |
| 5 mM | 0.2181 mL | 1.0903 mL | 2.1807 mL | 4.3614 mL | 5.4517 mL |
| 10 mM | 0.109 mL | 0.5452 mL | 1.0903 mL | 2.1807 mL | 2.7259 mL |
| 50 mM | 0.0218 mL | 0.109 mL | 0.2181 mL | 0.4361 mL | 0.5452 mL |
| 100 mM | 0.0109 mL | 0.0545 mL | 0.109 mL | 0.2181 mL | 0.2726 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Epitaxifolin
Catalog No.:BCX1062
CAS No.:153666-25-2
- Paeoniflorin sulfite
Catalog No.:BCX1061
CAS No.:1146967-98-7
- 2'-Fucosyllactose
Catalog No.:BCX1060
CAS No.:41263-94-9
- Myriacetin
Catalog No.:BCX1059
CAS No.:203734-35-4
- Phloretin 2'-xyloglucoside
Catalog No.:BCX1058
CAS No.:145758-09-4
- 5-Hydroxyferulic acid
Catalog No.:BCX1057
CAS No.:1782-55-4
- 3-Fucosyllactose
Catalog No.:BCX1056
CAS No.:41312-47-4
- Isohanalpinone
Catalog No.:BCX1055
CAS No.:103476-95-5
- Paeonidanin
Catalog No.:BCX1054
CAS No.:209969-75-5
- Isotheaflavin
Catalog No.:BCX1053
CAS No.:31701-93-6
- 11-Methoxyyangonin
Catalog No.:BCX1052
CAS No.:2743-14-8
- Phenaxolactone 1
Catalog No.:BCX1051
CAS No.:147022-96-6
- Podecdysone B
Catalog No.:BCX1064
CAS No.:22612-27-7
- Crocetine dimethyl ester
Catalog No.:BCX1065
CAS No.:5892-54-6
- Resveratrol 12-C-β-glucopyranoside
Catalog No.:BCX1066
CAS No.:163527-00-2
- Cochinchinenin A
Catalog No.:BCX1067
CAS No.:1057666-04-2
- trans-p-Coumaric acid 4-O-β-D-glucopyranoside
Catalog No.:BCX1068
CAS No.:117405-49-9
- Tamarixetin-3-O-rutinoside
Catalog No.:BCX1069
CAS No.:20550-05-4
- 2-O-α-D-Glucopyranosyl-L-ascorbic acid
Catalog No.:BCX1070
CAS No.:129499-78-1
- Stachyanthuside A
Catalog No.:BCX1071
CAS No.:864779-30-6
- Prunetinoside
Catalog No.:BCX1072
CAS No.:89595-66-4
- Momilacton B
Catalog No.:BCX1073
CAS No.:51415-08-8
- Momilacton A
Catalog No.:BCX1074
CAS No.:51415-07-7
- γ-Tocopherol
Catalog No.:BCX1075
CAS No.:54-28-4
Chemical transformation and target preparation of saponins in stems and leaves of Panax notoginseng.[Pubmed:29983608]
J Ginseng Res. 2018 Jul;42(3):270-276.
BACKGROUND: Notoginsenoside Ft1 is a promising potential candidate for cardiovascular and cancer disease therapy owing to its positive pharmacological activities. However, the yield of Ft1 is ultralow utilizing reported methods. Herein, an acid hydrolyzing strategy was implemented in the acquirement of rare notoginsenoside Ft1. METHODS: Chemical profiles were identified by ultraperformance liquid chromatography coupled with quadruple-time-of-flight and electrospray ionization mass spectrometry (UPLC-Q/TOF-ESI-MS). The acid hydrolyzing dynamic changes of chemical compositions and the possible transformation pathways of saponins were monitored by ultrahigh-performance LC coupled with tandem MS (UHPLC-MS/MS). RESULTS AND CONCLUSION: Notoginsenoside Ft1 was epimerized from Notoginsenoside ST4, which was generated through cleaving the carbohydrate side chains at C-20 of notoginsenosides Fa and Fc, and vina-ginsenoside R7, and further converted to other compounds via hydroxylation at C-25 or hydrolysis of the carbohydrate side chains at C-3 under the acid conditions. High temperature contributed to the hydroxylation reaction at C-25 and 25% acetic acid concentration was conducive to the preparation of notoginsenoside Ft1. C-20 epimers of notoginsenoside Ft1 and ST4 were successfully separated utilizing solvent method of acetic acid solution. The theoretical preparation yield rate of notoginsenoside Ft1 was about 1.8%, which would be beneficial to further study on its bioactivities and clinical application.


