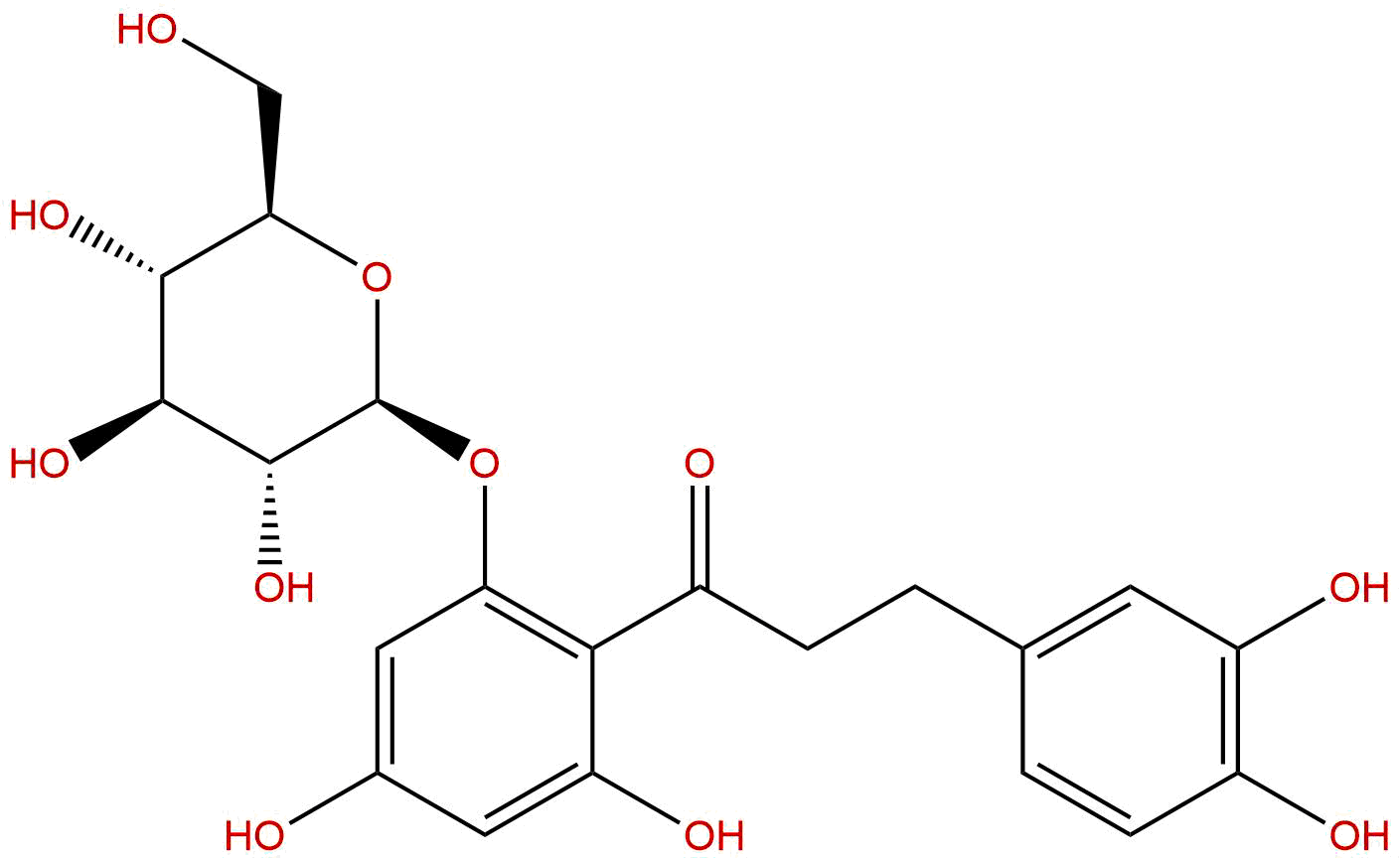
3-HydroxyphloridzinCAS# 30779-02-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 30779-02-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C21H24O11 | M.Wt | 452.41 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-Hydroxyphloridzin Dilution Calculator

3-Hydroxyphloridzin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2104 mL | 11.0519 mL | 22.1038 mL | 44.2077 mL | 55.2596 mL |
| 5 mM | 0.4421 mL | 2.2104 mL | 4.4208 mL | 8.8415 mL | 11.0519 mL |
| 10 mM | 0.221 mL | 1.1052 mL | 2.2104 mL | 4.4208 mL | 5.526 mL |
| 50 mM | 0.0442 mL | 0.221 mL | 0.4421 mL | 0.8842 mL | 1.1052 mL |
| 100 mM | 0.0221 mL | 0.1105 mL | 0.221 mL | 0.4421 mL | 0.5526 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Clenbuterol Impurity B
Catalog No.:BCX1098
CAS No.:37148-49-5
- Ginsenoside Rs1
Catalog No.:BCX1097
CAS No.:87733-67-3
- 5-O-Sinapoylshikimic acid
Catalog No.:BCX1096
CAS No.:2704629-41-2
- 5-O-Coumaroylshikimic acid
Catalog No.:BCX1095
CAS No.:111614-47-2
- 5-O-Feruloylshikimic acid
Catalog No.:BCX1094
CAS No.:1338228-73-1
- 5-O-(3,4-dimethoxycinnamoyl)shikimic acid
Catalog No.:BCX1093
CAS No.:1338228-77-5
- Methyl lucidenate F
Catalog No.:BCX1092
CAS No.:98665-10-2
- 3-O-Coumaroylquinic acid
Catalog No.:BCX1091
CAS No.:1899-30-5
- 4'-O-methylether-Homoeriodictyol 7-glucoside
Catalog No.:BCX1090
CAS No.:1612225-01-0
- 4'-O-methyl-Chlorogenic acid
Catalog No.:BCX1089
CAS No.:57496-29-4
- 4'-O-methyl-Neochlorogenic acid
Catalog No.:BCX1088
CAS No.:1234369-77-7
- Avenanthramide E
Catalog No.:BCX1087
CAS No.:93755-77-2
- 4-p-Coumaroylquinicacid
Catalog No.:BCX1100
CAS No.:1108200-72-1
- 20(R)-25-Methoxyprotopanaxadiol
Catalog No.:BCX1101
CAS No.:1050479-86-1
- Gypenoside
Catalog No.:BCX1102
CAS No.:862286-47-3
- Gypenoside
Catalog No.:BCX1103
CAS No.:862286-45-1
- Notoginsenoside N
Catalog No.:BCX1104
CAS No.:350586-56-0
- Notoginsenoside M
Catalog No.:BCX1105
CAS No.:394246-74-3
- Notoginsenoside FP1
Catalog No.:BCX1106
CAS No.:1004988-73-1
- Disodium uridine diphosphoglucose
Catalog No.:BCX1107
CAS No.:28053-08-9
- 4-(Acetyloxy)benzeneethanol
Catalog No.:BCX1108
CAS No.:60037-43-6
- Hyperforin acetate
Catalog No.:BCX1109
CAS No.:68324-06-1
- Benzoyltropein
Catalog No.:BCX1110
CAS No.:19145-60-9
- Maltooctaose
Catalog No.:BCX1111
CAS No.:66567-45-1
Enzyme-Assisted Supercritical Fluid Extraction of Flavonoids from Apple Pomace (Malusxdomestica).[Pubmed:38084785]
ChemSusChem. 2024 Apr 8;17(7):e202301094.
Herein an enzyme-assisted supercritical fluid extraction (EA-SFE) was developed using the enzyme mix snailase to obtain flavonols and dihydrochalcones, subgroups of flavonoids, from globally abundant waste product apple pomace. Snailase, a commercially available mix of 20-30 enzymes, was successfully used to remove the sugar moieties from quercetin glycosides, kaempferol glycosides, phloridzin and 3-Hydroxyphloridzin. The resulting flavonoid aglycones quercetin, kaempferol, phloretin and 3-hydroxyphloretin were extracted using supercritical carbon dioxide (scCO(2)) and minimum amounts of polar cosolvents. A sequential process of enzymatic hydrolysis and supercritical fluid extraction was developed, and the influence of the amount of snailase, pre-treatment of apple pomace, the time for enzymatic hydrolysis, the amount and type of cosolvent and the time for extraction, was studied. This revealed that even small amounts of snailase (0.25 %) provide a successful cleavage of sugar moieties up to 96 % after 2 h of enzymatic hydrolysis followed by supercritical fluid extraction with small amounts of methanol as cosolvent, leading up to 90 % of the total extraction yields after 1 h extraction time. Ultimately, a simultaneous process of EA-SFE successfully demonstrates the potential of snailase in scalable scCO(2) extraction processes for dry and wet apple pomace with satisfactory enzyme activity, even under pressurized conditions.
Molecular and Enzymatic Characterization of Flavonoid 3'-Hydroxylase of Malus x domestica.[Pubmed:34579488]
Plants (Basel). 2021 Sep 19;10(9):1956.
Malus x domestica (apple) accumulates particularly high amounts of dihydrochalcones in various tissues, with phloridzin (phloretin 2'-O-glucoside) being prevalent, although small amounts of 3-hydroxyphloretin and 3-Hydroxyphloridzin are also constitutively present. The latter was shown to correlate with increased disease resistance of transgenic M. x domestica plants. Two types of enzymes could be involved in 3-hydroxylation of dihydrochalcones: polyphenol oxidases or the flavonoid 3'-hydroxylase (F3'H), which catalyzes B-ring hydroxylation of flavonoids. We isolated two F3'H cDNA clones from apple leaves and tested recombinant Malus F3'Hs for their substrate specificity. From the two isolated cDNA clones, only F3'HII encoded a functionally active enzyme. In the F3'HI sequence, we identified two putatively relevant amino acids that were exchanged in comparison to that of a previously published F3'HI. Site directed mutagenesis, which exchanged an isoleucine into methionine in position 211 restored the functional activity, which is probably because it is located in an area involved in interaction with the substrate. In contrast to high activity with various flavonoid substrates, the recombinant enzymes did not accept phloretin under assay conditions, making an involvement in the dihydrochalcone biosynthesis unlikely.
Dihydrochalcone-derived polyphenols from tea crab apple (Malus hupehensis) and their inhibitory effects on alpha-glucosidase in vitro.[Pubmed:31070208]
Food Funct. 2019 May 22;10(5):2881-2887.
Three dihydrochalcone-derived polyphenols, huperolides A-C (1-3), along with thirteen known compounds (4-16) were isolated from the leaves of Malus hupehensis, the well-known tea crab apple in China. Their chemical structures were elucidated by extensive spectroscopic analysis including NMR (HSQC, HMBC, 1H-1H COSY and ROESY), HRMS and CD spectra. Huperolide A is a polyphenol with a new type of carbon skeleton, while huperolides B and C are a couple of atropisomers, which were isolated from natural sources for the first time. The antihyperglycemic effects of the isolated compounds were evaluated based on assaying their inhibitory activities against alpha-glucosidase. As a result, phlorizin (4), 3-Hydroxyphloridzin (5), 3-O-coumaroylquinic acid (12) and beta-hydroxypropiovanillone (15) showed significant concentration-dependent inhibitory effects on alpha-glucosidase. Therefore, those compounds might be responsible for the antihyperglycemic effect of this herb, and are the most promising compounds to lead discovery of drugs against diabetes.
Transgenic apple plants overexpressing the chalcone 3-hydroxylase gene of Cosmos sulphureus show increased levels of 3-hydroxyphloridzin and reduced susceptibility to apple scab and fire blight.[Pubmed:26895335]
Planta. 2016 May;243(5):1213-24.
Overexpression of chalcone-3-hydroxylase provokes increased accumulation of 3-Hydroxyphloridzin in Malus . Decreased flavonoid concentrations but unchanged flavonoid class composition were observed. The increased 3-hydroxyphlorizin contents correlate well with reduced susceptibility to fire blight and scab. The involvement of dihydrochalcones in the apple defence mechanism against pathogens is discussed but unknown biosynthetic steps in their formation hamper studies on their physiological relevance. The formation of 3-hydroxyphloretin is one of the gaps in the pathway. Polyphenol oxidases and cytochrome P450 dependent enzymes could be involved. Hydroxylation of phloretin in position 3 has high similarity to the B-ring hydroxylation of flavonoids catalysed by the well-known flavonoid 3'-hydroxylase (F3'H). Using recombinant F3'H and chalcone 3-hydroxylase (CH3H) from Cosmos sulphureus we show that F3'H and CH3H accept phloretin to some extent but higher conversion rates are obtained with CH3H. To test whether CH3H catalyzes the hydroxylation of dihydrochalcones in planta and if this could be of physiological relevance, we created transgenic apple trees harbouring CH3H from C. sulphureus. The three transgenic lines obtained showed lower polyphenol concentrations but no shift between the main polyphenol classes dihydrochalcones, flavonols, hydroxycinnamic acids and flavan 3-ols. Increase of 3-Hydroxyphloridzin within the dihydrochalcones and of epicatechin/catechin within soluble flavan 3-ols were observed. Decreased activity of dihydroflavonol 4-reductase and chalcone synthase/chalcone isomerase could partially explain the lower polyphenol concentrations. In comparison to the parent line, the transgenic CH3H-lines showed a lower disease susceptibility to fire blight and apple scab that correlated with the increased 3-hydroxyphlorizin contents.
UPLC-PDA quantification of chemical constituents of two different varieties (golden and royal) of apple leaves and their antioxidant activity.[Pubmed:25914106]
J Sci Food Agric. 2016 Mar 30;96(5):1440-50.
BACKGROUND: Malus domestica is the most widely cultivated fruit tree and is well known for its therapeutic value. Apple leaves are known to contain phenolic compounds but the nature of these has not been explored to the same extent as in apple fruit. A simple, rapid and sensitive ultra-performance liquid chromatography-diode array detection (UPLC-DAD) quantification method has been developed. Total polyphenol and flavonoid contents, as well as the antioxidant activity of golden and royal apple leaves were evaluated. RESULTS: Four compounds, namely rutin, 3-Hydroxyphloridzin, phloridzin and quercetin-3-O-arabinoside were identified by UPLC. The separation was achieved in less than 7 min. Total polyphenol and flavonoid contents were found to be slightly higher in apple golden variety than royal variety. The IC50 values determined by the DPPH assay were 49.94 microg mL(-1) for golden apple leaves and 43.89 microg mL(-1) for royal apple leaves. IC50 values determined by the ABTS assay were 47.10 and 66.53 microg mL(-1) for golden and royal apple leaves, respectively. Antioxidant activity was determined as 24.45 and 21.15 mg ascorbic acid g(-1) for golden and royal apple leaves, respectively, by using the FRAP assay. CONCLUSION: This study showed that apple leaves (both varieties) contain considerable amounts of polyphenols and flavonoids and are also a promising source of phloridzin.
High-speed counter-current chromatography coupled online to high performance liquid chromatography-diode array detector-mass spectrometry for purification, analysis and identification of target compounds from natural products.[Pubmed:25678319]
J Chromatogr A. 2015 Mar 13;1385:69-76.
A challenge in coupling high-speed counter-current chromatography (HSCCC) online with high performance liquid chromatography (HPLC) for purity analysis was their time incompatibility. Consequently, HSCCC-HPLC was conducted by either controlling HPLC analysis time and HSCCC flow rate or using stop-and-go scheme. For natural products containing compounds with a wide range of polarities, the former would optimize experimental conditions, while the latter required more time. Here, a novel HSCCC-HPLC-diode array detector-mass spectrometry (HSCCC-HPLC-DAD-MS) was developed for undisrupted purification, analysis and identification of multi-compounds from natural products. Two six-port injection valves and a six-port switching valve were used as interface for collecting key HSCCC effluents alternatively for HPLC-DAD-MS analysis and identification. The ethyl acetate extract of Malus doumeri was performed on the hyphenated system to verify its efficacy. Five main flavonoids, 3-Hydroxyphloridzin (1), phloridzin (2), 4',6'-dihydroxyhydrochalcone-2'-O-beta-D-glucopyranoside (3, first found in M. doumeri), phloretin (4), and chrysin (5), were purified with purities over 99% by extrusion elution and/or stepwise elution mode in two-step HSCCC, and 25mM ammonium acetate solution was selected instead of water to depress emulsification in the first HSCCC. The online system shortened manipulation time largely compared with off-line analysis procedure and stop-and-go scheme. The results indicated that the present method could serve as a simple, rapid and effective way to achieve target compounds with high purity from natural products.
Phenolic constituents of Malus doumeri var. formosana in the field of skin care.[Pubmed:16595910]
Biol Pharm Bull. 2006 Apr;29(4):740-5.
Plant phenolic compounds isolated from a 70% aqueous acetone extract of the leaves of Malus doumeri A. CHEV. var. formosana (KAWAK. & KOIDZ.) S. S. YING, a type of Taiwanese indigenous plant, were evaluated for potential application in the field of skin care. A phytochemical investigation of the active fractions resulted in the isolation of seven compounds of which the structures were identified by spectroscopic characterization. In the present study, the isolated phenolic compounds were evaluated for their free radical-scavenging activities against 1,1-diphenyl-2-picrylhydrazyl (DPPH) and the superoxide radicals, anti-elastase, and for their anti-matrix metalloproteinase-1 (MMP-1) activity in human skin fibroblast cells. Of these compounds, 3-Hydroxyphloridzin (2), 3-hydroxyphloretin (6), and quercetin (7) exhibited the strongest DPPH and superoxide radical-scavenging activities. The IC50 values of these compounds were 9.2, 7.7, and 15.4 microM, respectively, for the DPPH radical, and 25.0, 19.6, and 42.6 microM, respectively, for the superoxide radical. 3-Hydroxyphloridzin (2) and 3-hydroxyphloretin (6) also showed xanthine oxidase inhibitory activity, with IC(50) values of 52.1 and 22.4 muM, respectively. In the test for elastase inhibitory activity, phloretin (5) and 3-hydroxyphloretin (6) were the most potent compounds. Phloretin (5), 3-hydroxyphloretin (6), and quercetin (7) showed better inhibition of MMP-1 production in fibroblast cells. To the best of our knowledge, this is the first time that the active phenolic compounds from M. doumeri var. formosana have been isolated, reported, and described. The above results suggest that the extract of M. doumeri var. formosana containing phenolic compounds could be suitable naturally occurring active constituents for use in anti-aging or cosmetic products.


