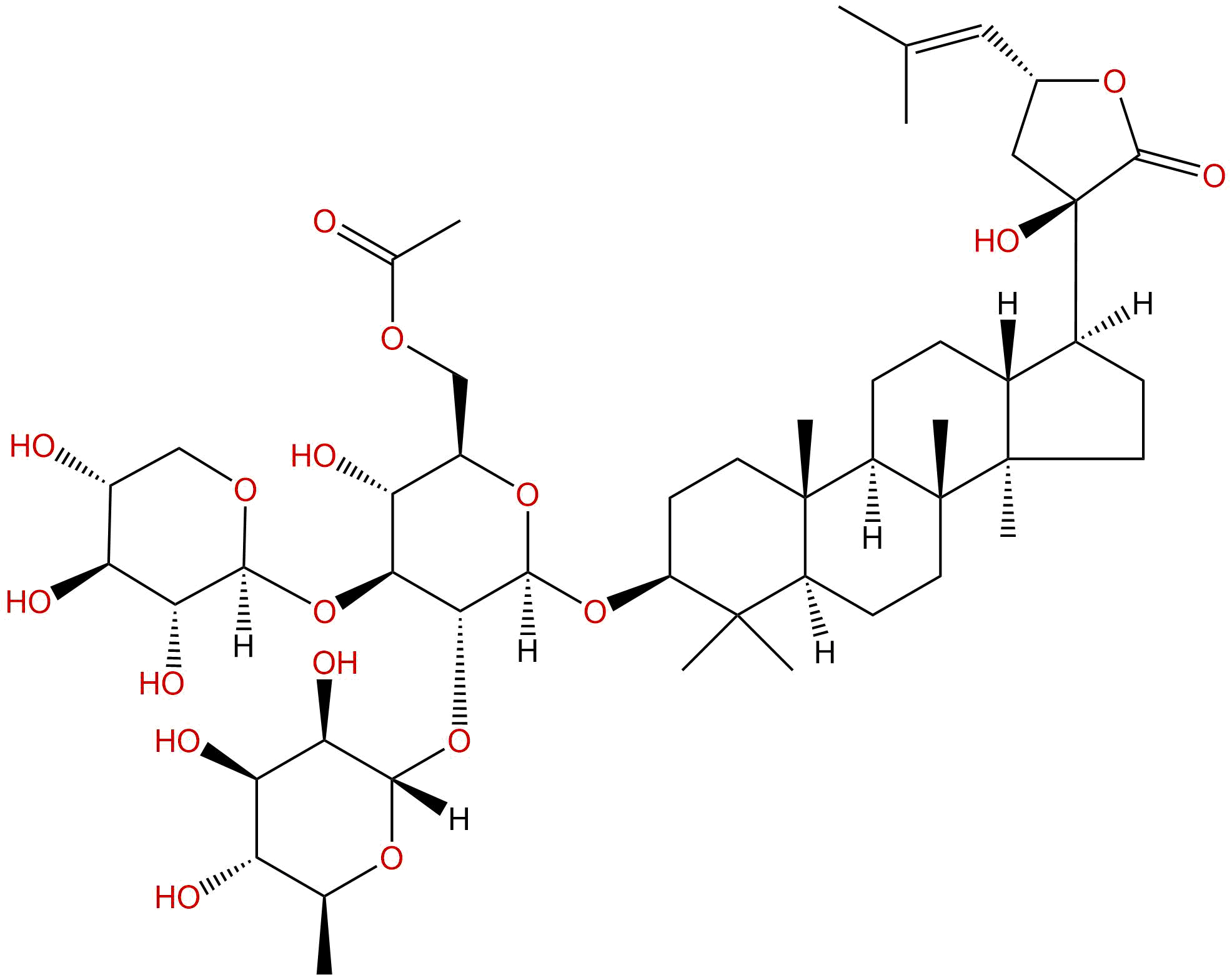
GypenosideCAS# 862286-47-3 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 862286-47-3 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C49H78O18 | M.Wt | 955.14 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Gypenoside Dilution Calculator

Gypenoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.047 mL | 5.2348 mL | 10.4697 mL | 20.9393 mL | 26.1742 mL |
| 5 mM | 0.2094 mL | 1.047 mL | 2.0939 mL | 4.1879 mL | 5.2348 mL |
| 10 mM | 0.1047 mL | 0.5235 mL | 1.047 mL | 2.0939 mL | 2.6174 mL |
| 50 mM | 0.0209 mL | 0.1047 mL | 0.2094 mL | 0.4188 mL | 0.5235 mL |
| 100 mM | 0.0105 mL | 0.0523 mL | 0.1047 mL | 0.2094 mL | 0.2617 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 20(R)-25-Methoxyprotopanaxadiol
Catalog No.:BCX1101
CAS No.:1050479-86-1
- 4-p-Coumaroylquinicacid
Catalog No.:BCX1100
CAS No.:1108200-72-1
- 3-Hydroxyphloridzin
Catalog No.:BCX1099
CAS No.:30779-02-3
- Clenbuterol Impurity B
Catalog No.:BCX1098
CAS No.:37148-49-5
- Ginsenoside Rs1
Catalog No.:BCX1097
CAS No.:87733-67-3
- 5-O-Sinapoylshikimic acid
Catalog No.:BCX1096
CAS No.:2704629-41-2
- 5-O-Coumaroylshikimic acid
Catalog No.:BCX1095
CAS No.:111614-47-2
- 5-O-Feruloylshikimic acid
Catalog No.:BCX1094
CAS No.:1338228-73-1
- 5-O-(3,4-dimethoxycinnamoyl)shikimic acid
Catalog No.:BCX1093
CAS No.:1338228-77-5
- Methyl lucidenate F
Catalog No.:BCX1092
CAS No.:98665-10-2
- 3-O-Coumaroylquinic acid
Catalog No.:BCX1091
CAS No.:1899-30-5
- 4'-O-methylether-Homoeriodictyol 7-glucoside
Catalog No.:BCX1090
CAS No.:1612225-01-0
- Gypenoside
Catalog No.:BCX1103
CAS No.:862286-45-1
- Notoginsenoside N
Catalog No.:BCX1104
CAS No.:350586-56-0
- Notoginsenoside M
Catalog No.:BCX1105
CAS No.:394246-74-3
- Notoginsenoside FP1
Catalog No.:BCX1106
CAS No.:1004988-73-1
- Disodium uridine diphosphoglucose
Catalog No.:BCX1107
CAS No.:28053-08-9
- 4-(Acetyloxy)benzeneethanol
Catalog No.:BCX1108
CAS No.:60037-43-6
- Hyperforin acetate
Catalog No.:BCX1109
CAS No.:68324-06-1
- Benzoyltropein
Catalog No.:BCX1110
CAS No.:19145-60-9
- Maltooctaose
Catalog No.:BCX1111
CAS No.:66567-45-1
- Maytansinol
Catalog No.:BCX1112
CAS No.:57103-68-1
- Irisolidone 7-O-glucoside
Catalog No.:BCX1113
CAS No.:126308-74-5
- Nifedipine impurity B
Catalog No.:BCX1114
CAS No.:50428-14-3
Tumor-targeted gypenoside nanodrug delivery system with double protective layers.[Pubmed:38687941]
J Cancer Res Ther. 2024 Apr 1;20(2):684-694.
OBJECTIVES: Gypenoside (Gyp) is easily degraded in the gastrointestinal tract, resulting in its low bioavailability. We aimed to develop a tumor-targeted Gyp nanodrug delivery system and to investigate its antitumor effect in vitro. MATERIALS AND METHODS: We used Gyp as the therapeutic drug molecule, mesoporous silica (MSN) and liposome (Lipo) as the drug carrier and protective layers, and aptamer SYL3C as the targeting element to establish a tumor-targeted nanodrug delivery system (i.e., SYL3C-Lipo@Gyp-MSN). The characteristics of SYL3C-Lipo@Gyp-MSN were investigated, and its drug release performance, cell uptake, and antitumor activity in vitro were evaluated. RESULTS: A tumor-targeted Gyp nanodrug delivery system was successfully prepared. The SYL3C-Lipo@Gyp-MSN was spherical or ellipsoidal; had good dispersion, which enabled it to specifically target and kill the liver tumor cell HepG2; and effectively protected the early leakage of Gyp. CONCLUSIONS: We have established a tumor-targeted nanodrug delivery system that can target and kill liver cancer cells and may provide a strategy for preparing new nanodrug-loaded preparations of traditional Chinese medicine.
The potential molecular mechanism underlying gypenoside amelioration of atherosclerosis in ApoE(-/-) mice: A multi-omics investigation.[Pubmed:38644881]
Heliyon. 2024 Apr 10;10(8):e29164.
Gypenosides (Gyp) are bioactive components of Gynostemma pentaphyllum that have a variety of pharmacological properties. Extracts of G. pentaphyllum have been found to be effective in the reduction of blood sugar and lipids and prevention of atherosclerosis. Here, the functions of Gyp and the mechanisms underlying their effects on atherosclerosis were investigated. Mice were allocated to three groups, namely, the control (C57BL/6), atherosclerosis model (ApoE(-/-) mice with high-fat diet), and Gyp-treated groups. Differentially expressed mRNAs, miRNAs, circRNA, and differential metabolites among the groups were analyzed. The results showed that "Fatty acid metabolism", "Fatty acid elongation", "Cytokine-cytokine receptor interaction", and "PI3K-Akt signaling pathway", amongst others, were involved in treatment process. Differentially expressed genes, including Fabp1, Apoe, FADS1, ADH1, SYNPO2, and Lmod1were also identified. Mmu-miR-30a and mmu-miR-30e showed reduced expression in atherosclerosis models but were increased following Gyp treatment, suggesting involvement in the effects of Gyp. In addition, chr5:150604177-150608440 were found to interact with mmu-miR-30a and mmu-miR-30e to regulate their abundance. In terms of metabolomics, Gyp may regulate biological processes involving PGD(2) and PGJ(2), potentially alleviating atherosclerosis. In conclusion, Gyp appeared to have complex effects on atherosclerosis, most of which were positive. These results support the use of Gyp in the treatment of atherosclerosis.
A promising therapy for fatty liver disease: PCSK9 inhibitors.[Pubmed:38547616]
Phytomedicine. 2024 Jun;128:155505.
BACKGROUND: Fatty liver disease (FLD) poses a significant global health concern worldwide, with its classification into nonalcoholic fatty liver disease (NAFLD) and alcoholic fatty liver disease (AFLD) contingent upon the presence or absence of chronic and excessive alcohol consumption. The absence of specific therapeutic interventions tailored to FLD at various stages of the disease renders its treatment exceptionally arduous. Despite the fact that FLD and hyperlipidemia are intimately associated, there is still debate over how lipid-lowering medications affect FLD. Proprotein Convertase Subtilisin/ Kexin type 9 (PCSK9) is a serine protease predominantly synthesized in the liver, which has a crucial impact on cholesterol homeostasis. Research has confirmed that PCSK9 inhibitors have prominent lipid-lowering properties and substantial clinical effectiveness, thereby justifying the need for additional exploration of their potential role in FLD. PURPOSE: Through a comprehensive literature search, this review is to identify the relationship and related mechanisms between PCSK9, lipid metabolism and FLD. Additionally, it will assess the pharmacological mechanism and applicability of PCSK9 inhibitors (including naturally occurring PCSK9 inhibitors, such as conventional herbal medicines) for the treatment of FLD and serve as a guide for updating the treatment protocol for such conditions. METHODS: A comprehensive literature search was conducted using several electronic databases, including Pubmed, Medline, Embase, CNKI, Wanfang database and ClinicalTrials.gov, from the inception of the database to 30 Jan 2024. Key words used in the literature search were "fatty liver", "hepatic steatosis", "PCSK9", "traditional Chinese medicine", "herb medicine", "botanical medicine", "clinical trial", "vivo", "vitro", linked with AND/OR. Most of the included studies were within five years. RESULTS: PCSK9 participates in the regulation of circulating lipids via both LDLR dependent and independent pathways, and there is a potential association with de novo lipogenesis. Major clinical studies have demonstrated a positive correlation between circulating PCSK9 levels and the severity of NAFLD, with elevated levels of circulating PCSK9 observed in individuals exposed to chronic alcohol. Numerous studies have demonstrated the potential of PCSK9 inhibitors to ameliorate non-alcoholic steatohepatitis (NASH), potentially completely alleviate liver steatosis, and diminish liver impairment. In animal experiments, PCSK9 inhibitors have exhibited efficacy in alleviating alcoholic induced liver lipid accumulation and hepatitis. Traditional Chinese medicine such as berberine, curcumin, resveratrol, piceatannol, sauchinone, lupin, quercetin, salidroside, ginkgolide, tanshinone, lunasin, Capsella bursa-pastoris, Gypenosides, and Morus alba leaves are the main natural PCS9 inhibitors. Excitingly, by inhibiting transcription, reducing secretion, direct targeting and other pathways, traditional Chinese medicine exert inhibitory effects on PCSK9, thereby exerting potential FLD therapeutic effects. CONCLUSION: PCSK9 plays an important role in the development of FLD, and PCSK9 inhibitors have demonstrated beneficial effects on lipid regulation and FLD in both preclinical and clinical studies. In addition, some traditional Chinese medicines have improved the disease progression of FLD by inhibiting PCSK9 and anti-inflammatory and antioxidant effects. Consequently, the inhibition of PCSK9 appears to be a promising therapeutic strategy for FLD.
Genome-wide characterization of the bHLH gene family in Gynostemma pentaphyllum reveals its potential role in the regulation of gypenoside biosynthesis.[Pubmed:38509465]
BMC Plant Biol. 2024 Mar 20;24(1):205.
BACKGROUND: Gynostemma pentaphyllum, an ancient Chinese herbal medicine, serves as a natural source of Gypenosides with significant medicinal properties. Basic helix-loop-helix (bHLH) transcription factors play pivotal roles in numerous biological processes, especially in the regulation of secondary metabolism in plants. However, the characteristics and functions of the bHLH genes in G. pentaphyllum remain unexplored, and their regulatory role in Gypenoside biosynthesis remains poorly elucidated. RESULTS: This study identified a total of 111 bHLH members in G. pentaphyllum (GpbHLHs), categorizing them into 26 subgroups based on shared conserved motif compositions and gene structures. Collinearity analysis illustrated that segmental duplications predominately lead to the evolution of GpbHLHs, with most duplicated GpbHLH gene pairs undergoing purifying selection. Among the nine Gypenoside-related GpbHLH genes, two GpbHLHs (GpbHLH15 and GpbHLH58) were selected for further investigation based on co-expression analysis and functional prediction. The expression of these two selected GpbHLHs was dramatically induced by methyl jasmonate, and their nuclear localization was confirmed. Furthermore, yeast one-hybrid and dual-luciferase assays demonstrated that GpbHLH15 and GpbHLH58 could bind to the promoters of the Gypenoside biosynthesis pathway genes, such as GpFPS1, GpSS1, and GpOSC1, and activate their promoter activity to varying degrees. CONCLUSIONS: In conclusion, our findings provide a detailed analysis of the bHLH family and valuable insights into the potential use of GpbHLHs to enhance the accumulation of Gypenosides in G. pentaphyllum.
Gypenoside XLIX alleviates acute liver injury: Emphasis on NF-kappaB/PPAR-alpha/NLRP3 pathways.[Pubmed:38503011]
Int Immunopharmacol. 2024 Apr 20;131:111872.
Liver is one of the vital organs in the human body and liver injury will have a very serious impact on human damage. Gypenoside XLIX is a PPAR-alpha activator that inhibits the activation of the NF-kappaB signaling pathway. The components of XLIX have pharmacological effects such as cardiovascular protection, antihypoxia, anti-tumor and anti-aging. In this study, we used cecum ligation and puncture (CLP) was used to induce in vivo mice hepatic injury, and lipopolysaccharide (LPS)-induced inflammation in RAW264.7 cells, evaluated whether Gypenoside XLIX could have a palliative effect on sepsis-induced acute liver injury via NF-kappaB/PPAR-alpha/NLRP3. In order to gain insight into these mechanisms, six groups were created in vivo: the Contol group, the Sham group, the CLP group, the CLP + XLIX group (40 mg/kg) and the Sham + XLIX (40 mg/kg) group, and the CLP + DEX (2 mg/kg) group. Three groups were created in vitro: Control, LPS, LPS + XLIX (40 muM). The analytical methods used included H&E staining, qPCR, reactive oxygen species (ROS), oil red O staining, and Western Blot. The results showed that XLIX attenuated hepatic inflammatory injury in mice with toxic liver disease through inhibition of the TLR4-mediated NF-kappaB pathway, attenuated lipid accumulation through activation of PPAR-alpha, and attenuated hepatic pyroptosis by inhibiting NLRP3 production. Regarding the imbalance between oxidative and antioxidant defenses due to septic liver injury, XLIX reduced liver oxidative stress-related biomarkers (ALT, AST), reduced ROS accumulation, decreased the amount of malondialdehyde (MDA) produced by lipid peroxidation, and increased the levels of antioxidant enzymes such as glutathione (GSH) and catalase (CAT). Our results demonstrate that XLIX can indeed attenuate septic liver injury. This is extremely important for future studies on XLIX and sepsis, and provides a potential pathway for the treatment of acute liver injury.
Hypolipidemic effect and gut microbiota regulation of Gypenoside aglycones in rats fed a high-fat diet.[Pubmed:38499259]
J Ethnopharmacol. 2024 Jun 28;328:118066.
ETHNOPHARMACOLOGICAL RELEVANCE: Gynostemma pentaphyllum (Thunb.) Makino has traditional applications in Chinese medicine to treat lipid abnormalities. Gypenosides (GPs), the main bioactive components of Gynostemma pentaphyllum, have been reported to exert hypolipidemic effects through multiple mechanisms. The lipid-lowering effects of GPs may be attributed to the aglycone portion resulting from hydrolysis of GPs by the gut microbiota. However, to date, there have been no reports on whether Gypenoside aglycones (Agl), the primary bioactive constituents, can ameliorate hyperlipidemia by modulating the gut microbiota. AIM OF THE STUDY: This study explored the potential therapeutic effects of Gypenoside aglycone (Agl) in a rat model of high-fat diet (HFD)-induced hyperlipidemia. METHODS: A hyperlipidemic rat model was established by feeding rats with a high-fat diet. Agl was administered orally, and serum lipid levels were analyzed. Molecular techniques, including RT-polymerase chain reaction (PCR) and fecal microbiota sequencing, were used to investigate the effects of Agl on lipid metabolism and gut microbiota composition. RESULTS: Agl administration significantly reduced serum levels of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) and mitigated hepatic damage induced by HFD. Molecular investigations have revealed the modulation of key lipid metabolism genes and proteins by Agl. Notably, Agl treatment enriched the gut microbiota with beneficial genera, including Lactobacillus, Akkermansia, and Blautia and promoted specific shifts in Lactobacillus murinus, Firmicutes bacterium CAG:424, and Allobaculum stercoricanis. CONCLUSION: This comprehensive study established Agl as a promising candidate for the treatment of hyperlipidemia. It also exhibits remarkable hypolipidemic and hepatoprotective properties. The modulation of lipid metabolism-related genes, along with the restoration of gut microbiota balance, provides mechanistic insights. Thus, Agl has great potential for clinical applications in hyperlipidemia management.
Multidimensional autophagy nano-regulator boosts Alzheimer's disease treatment by improving both extra/intraneuronal homeostasis.[Pubmed:38486986]
Acta Pharm Sin B. 2024 Mar;14(3):1380-1399.
Intraneuronal dysproteostasis and extraneuronal microenvironmental abnormalities in Alzheimer's disease (AD) collectively culminate in neuronal deterioration. In the context of AD, autophagy dysfunction, a multi-link obstacle involving autophagy downregulation and lysosome defects in neurons/microglia is highly implicated in intra/extraneuronal pathological processes. Therefore, multidimensional autophagy regulation strategies co-manipulating "autophagy induction" and "lysosome degradation" in dual targets (neuron and microglia) are more reliable for AD treatment. Accordingly, we designed an RP-1 peptide-modified reactive oxygen species (ROS)-responsive micelles (RT-NM) loading rapamycin or Gypenoside XVII. Guided by RP-1 peptide, the ligand of receptor for advanced glycation end products (RAGE), RT-NM efficiently targeted neurons and microglia in AD-affected region. This nano-combination therapy activated the whole autophagy-lysosome pathway by autophagy induction (rapamycin) and lysosome improvement (Gypenoside XVII), thus enhancing autophagic degradation of neurotoxic aggregates and inflammasomes, and promoting Abeta phagocytosis. Resultantly, it decreased aberrant protein burden, alleviated neuroinflammation, and eventually ameliorated memory defects in 3 x Tg-AD transgenic mice. Our research developed a multidimensional autophagy nano-regulator to boost the efficacy of autophagy-centered AD therapy.
Gypenoside induces apoptosis by inhibiting the PI3K/AKT/mTOR pathway and enhances T-cell antitumor immunity by inhibiting PD-L1 in gastric cancer.[Pubmed:38482051]
Front Pharmacol. 2024 Feb 28;15:1243353.
Introduction: Gypenoside is a natural extract of Gynostemma pentaphyllum (Thunb.) Makino, a plant in the Cucurbitaceae family. It has been reported to have antitumor effects on the proliferation, migration and apoptosis of various types of cancer cells. However, the use of Gypenoside in the treatment of gastric cancer has not been studied. In the present study, we explored the therapeutic effect of Gypenoside on gastric cancer and the potential molecular mechanism. Methods and Results: Our results showed that Gypenoside induced apoptosis in HGC-27 and SGC-7901 cells in a time-dependent and dose-dependent manner. Network pharmacology analyses predicted that Gypenoside exerts its therapeutic effects through the PI3K/AKT/mTOR signaling pathway. Furthermore, molecular docking and western blot experiments confirmed that Gypenoside induced the apoptosis of gastric cancer cells through the PI3K/AKT/mTOR signaling pathway. In addition, network pharmacological analysis revealed that the common targets of Gypenoside in gastric cancer were enriched in the immune effector process, PD-L1 expression, the PD-1 checkpoint pathway, and the Jak-STAT signaling pathway. Furthermore, molecular docking and western blot assays demonstrated that Gypenoside could bind to STAT3 and reduce its phosphorylation. Thus, the transcription of PD-L1 was inhibited in gastric cancer cells. Moreover, coculture experiments of gastric cancer cells with Gypenoside and primary mouse CD8(+) T cells showed that gastric cancer cells treated with Gypenoside could enhance the antitumor ability of T cells. Animal experiments confirmed the antitumor effect of Gypenoside, and the expression of PD-L1 was significantly downregulated in the Gypenoside-treated group. Conclusion: Gypenoside induced the apoptosis of gastric cancer cells by inhibiting the PI3K/AKT/mTOR pathway and simultaneously inhibited the expression of PD-L1 in gastric cancer cells, thus enhancing the antitumor immunity of T cells. This study provides a theoretical basis for applying Gypenoside as a new therapeutic agent to enhance the efficacy of immunotherapy in gastric cancer.
Natural Products in the Modulation of Farnesoid X Receptor Against Nonalcoholic Fatty Liver Disease.[Pubmed:38480498]
Am J Chin Med. 2024;52(2):291-314.
Nonalcoholic fatty liver disease (NAFLD) is a global health concern with a high prevalence and increasing economic burden, but official medicine remains unavailable. Farnesoid X receptor (FXR), a nuclear receptor member, is one of the most promising drug targets for NAFLD therapy that plays a crucial role in modulating bile acid, glucose, and lipid homeostasis, as well as inhibits hepatic inflammation and fibrosis. However, the rejection of the FXR agonist, obecholic acid, by the Food and Drug Administration for treating hepatic fibrosis raises a question about the functions of FXR in NAFLD progression and the therapeutic strategy to be used. Natural products, such as FXR modulators, have become the focus of attention for NAFLD therapy with fewer adverse reactions. The anti-NAFLD mechanisms seem to act as FXR agonists and antagonists or are involved in the FXR signaling pathway activation, indicating a promising target of FXR therapeutic prospects using natural products. This review discusses the effective mechanisms of FXR in NAFLD alleviation, and summarizes currently available natural products such as silymarin, glycyrrhizin, cycloastragenol, berberine, and Gypenosides, for targeting FXR, which can facilitate development of naturally targeted drug by medicinal specialists for effective treatment of NAFLD.
Gypenoside XIII regulates lipid metabolism in HepG2 hepatocytes and ameliorates nonalcoholic steatohepatitis in mice.[Pubmed:38294255]
Kaohsiung J Med Sci. 2024 Mar;40(3):280-290.
Gypenoside XIII is isolated from Gynostemma pentaphyllum (Thunb.) Makino. In mice, G. pentaphyllum extract and Gypenoside LXXV have been shown to improve non-alcoholic steatohepatitis (NASH). This study investigated whether Gypenoside XIII can regulate lipid accumulation in fatty liver cells or attenuate NASH in mice. We used HepG2 hepatocytes to establish a fatty liver cell model using 0.5 mM oleic acid. Fatty liver cells were treated with different concentrations of Gypenoside XIII to evaluate the molecular mechanisms of lipid metabolism. In addition, a methionine/choline-deficient diet induced NASH in C57BL/6 mice, which were given 10 mg/kg Gypenoside XIII by intraperitoneal injection. In fatty liver cells, Gypenoside XIII effectively suppressed lipid accumulation and lipid peroxidation. Furthermore, Gypenoside XIII significantly increased SIRT1 and AMPK phosphorylation to decrease acetyl-CoA carboxylase phosphorylation, reducing fatty acid synthesis activity. Gypenoside XIII also decreased lipogenesis by suppressing sterol regulatory element-binding protein 1c and fatty acid synthase production. Gypenoside XIII also increased lipolysis and fatty acid beta-oxidation by promoting adipose triglyceride lipase and carnitine palmitoyltransferase 1, respectively. In an animal model of NASH, Gypenoside XIII effectively decreased the lipid vacuole size and number and reduced liver fibrosis and inflammation. These findings suggest that Gypenoside XIII can regulate lipid metabolism in fatty liver cells and improve liver fibrosis in NASH mice. Therefore, Gypenoside XIII has potential as a novel agent for the treatment of NASH.
Gypenoside XLIX attenuates sepsis-induced splenic injury through inhibiting inflammation and oxidative stress.[Pubmed:38142642]
Int Immunopharmacol. 2024 Jan 25;127:111420.
BACKGROUND: To investigate the effect of Gypenoside XLIX (Gyp-XLIX) on acute splenic injury (ASI) induced by cecal ligation and puncture (CLP) in septic mice, a study was conducted. METHODS: Sixty healthy mice were randomly divided into six groups: the NC group, the Sham group, the Sham + Gyp-XLIX group, the CLP group, the CLP + Gyp-XLIX group, and the CLP + Dexamethasone (DEX) group. The NC group did not undergo any operation, while the rest of the groups underwent CLP to establish the sepsis model. The Sham group only underwent open-abdominal suture surgery without cecum puncture. After the operation, the groups were immediately administered the drug for a total of 5 days. Various methods such as hematoxylin and eosin (HE) staining, biochemical kits, qRT-PCR, and reactive oxygen species (ROS) were used for analysis. RESULTS: The results demonstrated that Gyp-XLIX effectively mitigated the splenic histopathological damage, while reducing the malondialdehyde (MDA) lipid peroxidation index and enhancing the antioxidant activities of catalase (CAT), glutathione (GSH) and total antioxidant capacity (T-AOC). The utilization of Dihydroethidium (DHE) fluorescent probe revealed that Gyp-XLIX inhibited the acute splenic accumulation of ROS induced by CLP in septic mice. Further investigations revealed that Gyp-XLIX exhibited a down-regulatory effect on the protein levels of inflammatory mediators iNOS and COX-2, consequently leading to the suppression of pro-inflammatory cytokines such as TNF-alpha, IL-6, and IL-1beta. Additionally, it up-regulated the expression of anti-inflammatory factor IL-10. CONCLUSION: In conclusion, Gyp-XLIX was significantly effective in attenuating CLP-induced acute splenic inflammation and oxidative stress in septic mice.
[Comparison on anti-inflammatory activity of Gynostemma pentaphyllum processed with different methods].[Pubmed:38114112]
Zhongguo Zhong Yao Za Zhi. 2023 Oct;48(19):5235-5243.
The aim of this study is to investigate the effects of Gynostemma pentaphyllum dried with two different methods(air drying and heating) on inflammation in acute lung injury(ALI) mice in vivo and in vitro. Lipopolysaccharide(LPS) was sprayed into the airway of wild type C57BL/6J male mice to establish the model, and the drug was injected into the tail vein 24 h after modeling. Lung function, lung tissue wet/dry weight(W/D) ratio, the total protein concentration, interleukin 6(IL-6), IL-1beta, and tumor necrosis factor-alpha(TNF-alpha) in the bronchoalveolar lavage fluid(BALF), and pathological changes of the lung tissue were used to evaluate the effects of different Gypenosides on ALI mice. The results showed that total Gypenosides(YGGPs) and the Gypenosides substituted with one or two glycosyl(GPs_(1-2)) in the air-dried sample improved the lung function, significantly lowered the levels of IL-1beta and TNF-alpha in BALF, and alleviated the lung inflammation of ALI mice. Moreover, GPs_(1-2) had a more significant effect on inhibiting NO release in RAW264.7 cells. This study showed that different drying methods affected the anti-inflammatory activity of G. pentaphyllum, and the rare saponins in the air-dried sample without heating had better anti-inflammatory activity.
Gypenoside IX restores Akt/GSK-3beta pathway and alleviates Alzheimer's disease-like neuropathology and cognitive deficits.[Pubmed:38095632]
Aging (Albany NY). 2023 Dec 12;15(23):14172-14191.
The main pathological changes of Alzheimer's disease (AD), a progressive neurodegenerative disorder, include senile plaque (deposited by amyloid beta), neurofibrillary tangle (formed by paired helical filaments composed of hyperphosphorylated tau), and massive loss of neurons. Currently there is a lack of ideal drugs to halt AD progression. Gypenosides (GPs), a kind of natural product, possesses potential therapeutic effects for neurodegenerative diseases, including AD. However, the specific role and mechanism of GPs for AD remain unclear. In the current study, we used staurosporine (STP), an inducer of apoptosis and causing tau hyperphosphorylation, to mimic AD models, and explored the role and mechanism of Gypenoside IX (one of the extracts of Gynostemma, GP for short name in our experiments) in STP treated primary hippocampal neurons and rats. We found STP not only increased apoptosis and tau hyperphosphorylation, but also significantly increased Abeta production, resulting in synaptic dysfunction and cognitive decline in mimic AD models by STP. GP was found to rescue apoptosis and cognitive impairments caused by STP treatment. Moreover, GP recovered the decreased synaptic proteins PSD95, Synaptophysin and GluR2, and blocked dendritic spine loss. Interestingly, GP decreased the STP induced tau hyperphosphorylation at different sites including S-199, S-202, T-205, T-231, S-262, S-396, and S-404, and at the same time decreased Abeta production through down-regulation of BACE1 and PS1. These effects in STP treated primary hippocampal neurons and rats were accompanied with a restoration of AKT/GSK-3beta signaling axis with GP treatment, supporting that dysregulation of AKT/GSK-3beta pathway might be involved in STP related AD pathogenesis. The results from our research proved that GP might be a potential candidate compound to reduce neuronal damage and prevent the cognitive decline in AD.


