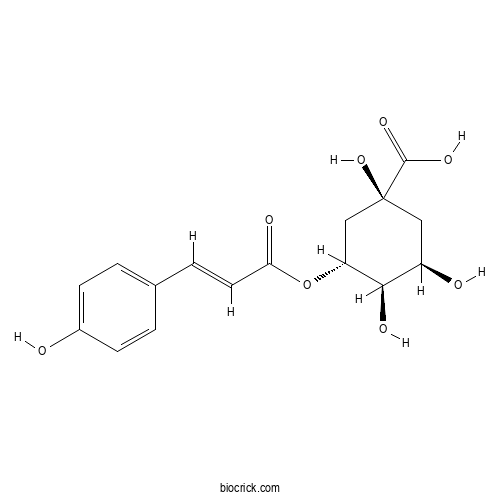
3-O-Coumaroylquinic acidCAS# 1899-30-5 |
- 5-O-Coumaroylquinic acid
Catalog No.:BCX0028
CAS No.:32451-86-8
- 3-O-Coumaroylquinic acid
Catalog No.:BCX0658
CAS No.:87099-71-6
- 3-O-p-Coumaroylquinic acid
Catalog No.:BCX0783
CAS No.:5746-55-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1899-30-5 | SDF | Download SDF |
| PubChem ID | 6441280.0 | Appearance | Powder |
| Formula | C16H18O8 | M.Wt | 338.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,3R,4R,5R)-1,3,4-trihydroxy-5-[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]oxycyclohexane-1-carboxylic acid | ||
| SMILES | C1C(C(C(CC1(C(=O)O)O)OC(=O)C=CC2=CC=C(C=C2)O)O)O | ||
| Standard InChIKey | BMRSEYFENKXDIS-OTCYKTEZSA-N | ||
| Standard InChI | InChI=1S/C16H18O8/c17-10-4-1-9(2-5-10)3-6-13(19)24-12-8-16(23,15(21)22)7-11(18)14(12)20/h1-6,11-12,14,17-18,20,23H,7-8H2,(H,21,22)/b6-3+/t11-,12-,14-,16+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-O-Coumaroylquinic acid Dilution Calculator

3-O-Coumaroylquinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9559 mL | 14.7793 mL | 29.5587 mL | 59.1174 mL | 73.8967 mL |
| 5 mM | 0.5912 mL | 2.9559 mL | 5.9117 mL | 11.8235 mL | 14.7793 mL |
| 10 mM | 0.2956 mL | 1.4779 mL | 2.9559 mL | 5.9117 mL | 7.3897 mL |
| 50 mM | 0.0591 mL | 0.2956 mL | 0.5912 mL | 1.1823 mL | 1.4779 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2956 mL | 0.5912 mL | 0.739 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4'-O-methylether-Homoeriodictyol 7-glucoside
Catalog No.:BCX1090
CAS No.:1612225-01-0
- 4'-O-methyl-Chlorogenic acid
Catalog No.:BCX1089
CAS No.:57496-29-4
- 4'-O-methyl-Neochlorogenic acid
Catalog No.:BCX1088
CAS No.:1234369-77-7
- Avenanthramide E
Catalog No.:BCX1087
CAS No.:93755-77-2
- Kuwanon W
Catalog No.:BCX1086
CAS No.:95518-95-9
- Indican
Catalog No.:BCX1085
CAS No.:487-60-5
- 3'-Sialyllactose Sodium Salt
Catalog No.:BCX1084
CAS No.:128596-80-5
- 6'-Sialyllactose Sodium Salt
Catalog No.:BCX1083
CAS No.:157574-76-0
- 6-Iodo Diosmin
Catalog No.:BCX1082
CAS No.:1431536-92-3
- Pseudostellarin B
Catalog No.:BCX1081
CAS No.:156430-21-6
- Rhamnetin 3-O-rutinoside
Catalog No.:BCX1080
CAS No.:34202-83-0
- 4-Deoxy-4α-phorbol
Catalog No.:BCX1079
CAS No.:37415-57-9
- Methyl lucidenate F
Catalog No.:BCX1092
CAS No.:98665-10-2
- 5-O-(3,4-dimethoxycinnamoyl)shikimic acid
Catalog No.:BCX1093
CAS No.:1338228-77-5
- 5-O-Feruloylshikimic acid
Catalog No.:BCX1094
CAS No.:1338228-73-1
- 5-O-Coumaroylshikimic acid
Catalog No.:BCX1095
CAS No.:111614-47-2
- 5-O-Sinapoylshikimic acid
Catalog No.:BCX1096
CAS No.:2704629-41-2
- Ginsenoside Rs1
Catalog No.:BCX1097
CAS No.:87733-67-3
- Clenbuterol Impurity B
Catalog No.:BCX1098
CAS No.:37148-49-5
- 3-Hydroxyphloridzin
Catalog No.:BCX1099
CAS No.:30779-02-3
- 4-p-Coumaroylquinicacid
Catalog No.:BCX1100
CAS No.:1108200-72-1
- 20(R)-25-Methoxyprotopanaxadiol
Catalog No.:BCX1101
CAS No.:1050479-86-1
- Gypenoside
Catalog No.:BCX1102
CAS No.:862286-47-3
- Gypenoside
Catalog No.:BCX1103
CAS No.:862286-45-1
Extraction, isolation and identification of four phenolic compounds from Pleioblastus amarus shoots and their antioxidant and anti-inflammatory properties in vitro.[Pubmed:34915365]
Food Chem. 2022 Apr 16;374:131743.
Pleioblastus amarus (P. amarus) shoots, belong to the grass family Gramineae, a traditional green vegetable in China, are rich in nutritional properties, and can provide various health benefits. This study isolated four compounds, namely (1-4), 3-O-Coumaroylquinic acid (1), 3-O-feruloylquinic acid (2), 4-O-feruloylquinic acid (3), and 5-O-feruloylquinic acid (4) from Pleioblastus amarus shoots for the first time. The structures of the extracted compounds were determined using detailed spectroscopic (1D/2D NMR), high resolution electrospray ionization mass spectrometry (HR-ESI-MS), and infrared (IR) spectroscopy. The antioxidant capacity of 3-O-feruloylquinic acid (2) was stronger than that of the other compounds, while it also exhibited anti-inflammatory activity, significantly restricting the release of nitric oxide (NO) by lipopolysaccharide (LPS)-induced RAW 264.7 cells, displaying an inhibitory rate of 60.92 percent at a concentration of 400 mug/mL. Furthermore, 3-O-feruloylquinic acid (2) inhibited interleukin-1beta (IL-1beta), interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and nuclear factor-kappaB (NF-kappaB) expression and may be useful for developing novel antioxidant and anti-inflammatory substances.
Dihydrochalcone-derived polyphenols from tea crab apple (Malus hupehensis) and their inhibitory effects on alpha-glucosidase in vitro.[Pubmed:31070208]
Food Funct. 2019 May 22;10(5):2881-2887.
Three dihydrochalcone-derived polyphenols, huperolides A-C (1-3), along with thirteen known compounds (4-16) were isolated from the leaves of Malus hupehensis, the well-known tea crab apple in China. Their chemical structures were elucidated by extensive spectroscopic analysis including NMR (HSQC, HMBC, 1H-1H COSY and ROESY), HRMS and CD spectra. Huperolide A is a polyphenol with a new type of carbon skeleton, while huperolides B and C are a couple of atropisomers, which were isolated from natural sources for the first time. The antihyperglycemic effects of the isolated compounds were evaluated based on assaying their inhibitory activities against alpha-glucosidase. As a result, phlorizin (4), 3-hydroxyphloridzin (5), 3-O-Coumaroylquinic acid (12) and beta-hydroxypropiovanillone (15) showed significant concentration-dependent inhibitory effects on alpha-glucosidase. Therefore, those compounds might be responsible for the antihyperglycemic effect of this herb, and are the most promising compounds to lead discovery of drugs against diabetes.
Identification of the major constituents of Hypericum perforatum by LC/SPE/NMR and/or LC/MS.[Pubmed:17196625]
Phytochemistry. 2007 Feb;68(3):383-93.
The newly established hyphenated instrumentation of LC/DAD/SPE/NMR and LC/UV/(ESI)MS techniques have been applied for separation and structure verification of the major known constituents present in Greek Hypericum perforatum extracts. The chromatographic separation was performed on a C18 column. Acetonitrile-water was used as a mobile phase. For the on-line NMR detection, the analytes eluted from column were trapped one by one onto separate SPE cartridges, and hereafter transported into the NMR flow-cell. LC/DAD/SPE/NMR and LC/UV/MS allowed the characterization of constituents of Greek H. perforatum, mainly naphtodianthrones (hypericin, pseudohypericin, protohypericin, protopseudohypericin), phloroglucinols (hyperforin, adhyperforin), flavonoids (quercetin, quercitrin, isoquercitrin, hyperoside, astilbin, miquelianin, I3,II8-biapigenin) and phenolic acids (chlorogenic acid, 3-O-Coumaroylquinic acid). Two phloroglucinols (hyperfirin and adhyperfirin) were detected for the first time, which have been previously reported to be precursors in the biosynthesis of hyperforin and adhyperforin.


