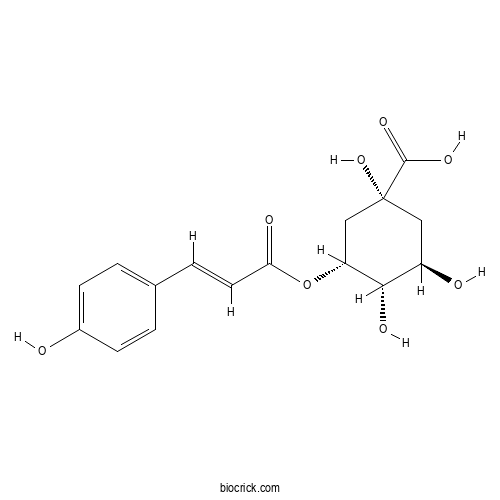
5-O-Coumaroylquinic acidCAS# 32451-86-8 |
- 3-O-Coumaroylquinic acid
Catalog No.:BCX0658
CAS No.:87099-71-6
- 3-O-p-Coumaroylquinic acid
Catalog No.:BCX0783
CAS No.:5746-55-4
- 3-O-Coumaroylquinic acid
Catalog No.:BCX1091
CAS No.:1899-30-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 32451-86-8 | SDF | Download SDF |
| PubChem ID | 9945785 | Appearance | Powder |
| Formula | C16H18O8 | M.Wt | 338.3 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1R,3R,4S,5R)-1,3,4-trihydroxy-5-[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]oxycyclohexane-1-carboxylic acid | ||
| SMILES | C1C(C(C(CC1(C(=O)O)O)OC(=O)C=CC2=CC=C(C=C2)O)O)O | ||
| Standard InChIKey | BMRSEYFENKXDIS-QHAYPTCMSA-N | ||
| Standard InChI | InChI=1S/C16H18O8/c17-10-4-1-9(2-5-10)3-6-13(19)24-12-8-16(23,15(21)22)7-11(18)14(12)20/h1-6,11-12,14,17-18,20,23H,7-8H2,(H,21,22)/b6-3+/t11-,12-,14+,16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

5-O-Coumaroylquinic acid Dilution Calculator

5-O-Coumaroylquinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.956 mL | 14.7798 mL | 29.5596 mL | 59.1191 mL | 73.8989 mL |
| 5 mM | 0.5912 mL | 2.956 mL | 5.9119 mL | 11.8238 mL | 14.7798 mL |
| 10 mM | 0.2956 mL | 1.478 mL | 2.956 mL | 5.9119 mL | 7.3899 mL |
| 50 mM | 0.0591 mL | 0.2956 mL | 0.5912 mL | 1.1824 mL | 1.478 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2956 mL | 0.5912 mL | 0.739 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gossypetin 3-sophoroside-8-glucoside
Catalog No.:BCX0027
CAS No.:77306-93-5
- Alopecurone A
Catalog No.:BCX0026
CAS No.:162558-89-6
- Kidjolanin
Catalog No.:BCX0025
CAS No.:38395-01-6
- Hainanic acid B
Catalog No.:BCX0024
CAS No.:1637737-46-2
- 5-Hydroxy-4a,8-dimethyl-3-methylen-decahydroazuleno[6,5-b]furan-2(3H)-on
Catalog No.:BCX0023
CAS No.:114579-31-6
- Ganoderic acid GS-3
Catalog No.:BCX0022
CAS No.:1206781-66-9
- Sophoraflavone A
Catalog No.:BCX0021
CAS No.:105594-08-9
- 12beta-Acetoxy-3,7,11,15,23-pentaoxo-lanost-8,20-dien-26-oic acid
Catalog No.:BCX0020
CAS No.:1309931-91-6
- erythro-Austrobailignan-6
Catalog No.:BCX0019
CAS No.:114127-24-1
- Herbacetin 3-sophoroside-8-glucoside
Catalog No.:BCX0018
CAS No.:77298-68-1
- Regaloside I
Catalog No.:BCX0017
CAS No.:126239-78-9
- Dihydroconfertin
Catalog No.:BCX0016
CAS No.:68832-40-6
- Catechin 7-O-beta-D-glucopyranoside
Catalog No.:BCX0029
CAS No.:65597-47-9
- Odontoside
Catalog No.:BCX0030
CAS No.:20300-50-9
- Caffeoylcalleryanin
Catalog No.:BCX0031
CAS No.:20300-49-6
- Ganolucidic acid E
Catalog No.:BCX0032
CAS No.:114567-50-9
- Apigenin-6-C-beta-D-glucopyranosyl-8-C-[alpha-L-rhamnopyranosyl-(1->2)]-beta-glucopyranoside
Catalog No.:BCX0033
CAS No.:1646598-06-2
- Tembetarine
Catalog No.:BCX0034
CAS No.:18446-73-6
- Scoparin
Catalog No.:BCX0035
CAS No.:301-16-6
- (Z)-Ferulic acid 4-O-beta-D-glucoside
Catalog No.:BCX0036
CAS No.:94942-20-8
- Methyl ganoderate A
Catalog No.:BCX0037
CAS No.:105742-78-7
- (3,4-Dihydroxyphenyl)methyl 3-(beta-D-glucopyranosyloxy)-4-hydroxybenzoate
Catalog No.:BCX0038
CAS No.:877461-90-0
- Bayin
Catalog No.:BCX0039
CAS No.:3681-96-7
- Ganoderic acid GS-2
Catalog No.:BCX0040
CAS No.:1206781-65-8
Flavonoids from Hypericum patulum enhance glucose consumption and attenuate lipid accumulation in HepG2 cells.[Pubmed:34378802]
J Food Biochem. 2021 Sep;45(9):e13898.
Hypericum patulum has been used as a folk medicine for its varied therapeutic effects including antifungal, wound-healing, spasmolytic, stimulant, hypotensive activities. The water decoction is drank as tea could treat cold, infantile malnutrition. The present study aims to isolate the constituents of the plant and investigate their effects on the glucose consumption in insulin-resistant HepG2 cells, furthermore, lipid metabolism in oleic acid (OA)-treated HepG2 cells was also studied. The phytochemical investigation of the plant led to the isolation of eleven compounds, and their structures were identified by spectroscopic analysis as n-dotriacontanol (1), shikimic acid (2), 1-O-caffeoylquinic acid methyl ester (3), 5-O-caffeoylquinic acid methyl ester (4), 5-O-Coumaroylquinic acid methyl ester (5), 5-O-caffeoylquinic acid butyl ester (6), quercetin-3-O-alpha-L-rhamnoside (7), quercetin (8), quercetin-3-O-(4-methoxy)-alpha-L-rahmnopyranosyl (9), hyperoside (10), and rutin (11). The results revealed that compounds 7, 9, and 10 could enhance glucose consumption significantly in hyperglycemia induced HepG2 cells and insulin-resistant HepG2 cells. In addition, the western blotting analysis result exhibited that compounds 7, 9, and 10 in high concentration (5 muM, H) group could dramatically upregulate the expression of PPARgamma protein, and even the effect of them had no significant difference compared with that of rosiglitazone. Furthermore, compounds 9 and 10 in middle concentration (2.5 muM, M) group and H group could dramatically promote triglyceride metabolism and decrease TG content in OA-treated HepG2 cells, and even in H group, reactive oxygen species (ROS) level were significantly decreased compared with model group. PRACTICAL APPLICATIONS: Hypericum patulum is a well-known plant of the genera Hypericum for its varied preventive and therapeutic potential activities. To study the chemical constituents and their effects on glucose and lipid metabolism in vitro, we detected glucose consumption in insulin-resistant HepG2 cells, triglyceride content and reactive oxygen species level in OA-treated HepG2 cells. In addition, PPARgamma protein was also detected by western blotting analysis in the study. Compounds 1, 2, 3, 5, 6, 9, 10, and 11 were isolated from the plant for the first time. Quercetin-3-O-(4"-methoxy)-alpha-L-rahmnopyranosyl (9) and hyperoside (10) had potential therapeutic benefit against glucose and lipid metabolic disease. Therefore, this study might have certain guiding significance for further research and development of H. patulum.
Simultaneous Determination of 10 Bioactive Components of Lophatherum gracile Brongn by HPLC-DAD.[Pubmed:25527702]
J Chromatogr Sci. 2015 Jul;53(6):963-7.
A high-performance liquid chromatography method coupled with diode array detection (HPLC-DAD) was developed for simultaneous determination of two coumarins and eight flavonoids in Lophatherum gracile Brongn (Gramineae), namely 5-O-Coumaroylquinic acid (i), 4-O-coumaroylquinic acid (ii), luteolin 6-C-beta-d-galactopyranosiduronic acid (1-->2)-beta-d-glucopyranoside (iii), 7-O-beta-d-glucopyranosyl-6-C-alpha-l-arabinopy ranoside (iv), isoorientin (v), swertiajaponin (vi), luteolin 6-C-beta-d-galactopyranosiduronic acid (1-->2)-alpha-l-arabinopyranoside (vii), Saponaretin (viii), swertisin (ix) and apigenin 6-C-beta-d-galactopyranosiduronic acid (1-->2)-alpha-l-arabinopyranoside (x). The analysis was performed on Cosmosil MS-II C18 column (250 x 4.6 mm, 5 microm) with gradient elution of 0.1% aqueous acetic acid and acetonitrile. The detection wavelength was 330 nm. The developed method was able to determine the bioactive compounds with excellent resolution, precision and recovery. The validated method was successfully applied for the analysis of the 10 bioactive compounds in n samples from different cultivated regions. The results indicated that the developed method can be used as a suitable quality control method for L. gracile.


