3-O-p-Coumaroylquinic acidCAS# 5746-55-4 |
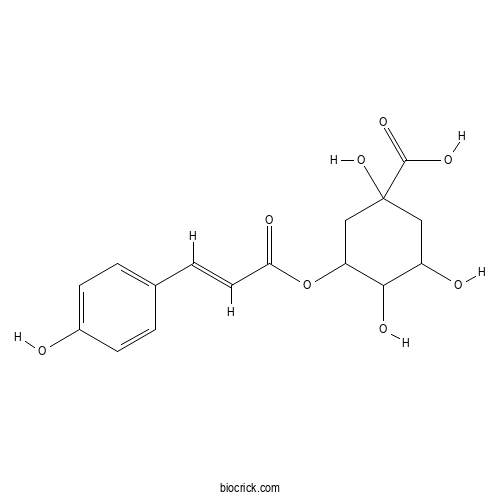
- 5-O-Coumaroylquinic acid
Catalog No.:BCX0028
CAS No.:32451-86-8
- 3-O-Coumaroylquinic acid
Catalog No.:BCX0658
CAS No.:87099-71-6
- 3-O-Coumaroylquinic acid
Catalog No.:BCX1091
CAS No.:1899-30-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5746-55-4 | SDF | Download SDF |
| PubChem ID | 14158101.0 | Appearance | Powder |
| Formula | C16H18O8 | M.Wt | 338.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,3,4-trihydroxy-5-[(E)-3-(4-hydroxyphenyl)prop-2-enoyl]oxycyclohexane-1-carboxylic acid | ||
| SMILES | C1C(C(C(CC1(C(=O)O)O)OC(=O)C=CC2=CC=C(C=C2)O)O)O | ||
| Standard InChIKey | BMRSEYFENKXDIS-ZZXKWVIFSA-N | ||
| Standard InChI | InChI=1S/C16H18O8/c17-10-4-1-9(2-5-10)3-6-13(19)24-12-8-16(23,15(21)22)7-11(18)14(12)20/h1-6,11-12,14,17-18,20,23H,7-8H2,(H,21,22)/b6-3+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

3-O-p-Coumaroylquinic acid Dilution Calculator

3-O-p-Coumaroylquinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9559 mL | 14.7793 mL | 29.5587 mL | 59.1174 mL | 73.8967 mL |
| 5 mM | 0.5912 mL | 2.9559 mL | 5.9117 mL | 11.8235 mL | 14.7793 mL |
| 10 mM | 0.2956 mL | 1.4779 mL | 2.9559 mL | 5.9117 mL | 7.3897 mL |
| 50 mM | 0.0591 mL | 0.2956 mL | 0.5912 mL | 1.1823 mL | 1.4779 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2956 mL | 0.5912 mL | 0.739 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bixin
Catalog No.:BCX0782
CAS No.:6983-79-5
- Frangufoline
Catalog No.:BCX0781
CAS No.:19526-09-1
- Malonic acid
Catalog No.:BCX0780
CAS No.:90844-16-9
- Isogermafurenolide
Catalog No.:BCX0779
CAS No.:20267-89-4
- Nardoguaianone J
Catalog No.:BCX0778
CAS No.:443128-64-1
- Verrucosin
Catalog No.:BCX0777
CAS No.:83198-63-4
- Maltotriose
Catalog No.:BCX0776
CAS No.:1109-28-0
- 20-Deoxy,5-benzoyl-Ingenol
Catalog No.:BCX0775
CAS No.:54706-97-7
- 2,3-dihydroxypropyl 9-octadecenoate
Catalog No.:BCX0774
CAS No.:251983-54-7
- Nardoguaianone K
Catalog No.:BCX0773
CAS No.:443128-65-2
- 2-Hydroxycinnamicaldehyde
Catalog No.:BCX0772
CAS No.:3541-42-2
- (Z)-9-Nonadecene
Catalog No.:BCX0771
CAS No.:51865-02-2
- Choerospondin
Catalog No.:BCX0784
CAS No.:81202-36-0
- Myricetin 3'-methyl-3-O-rutinoside
Catalog No.:BCX0785
CAS No.:55481-90-8
- Verbascose
Catalog No.:BCX0786
CAS No.:546-62-3
- Chrysotoxine
Catalog No.:BCX0787
CAS No.:156951-82-5
- 3-[[6-Deoxy-2-O-[6-O-[3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]-α-L-mannopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-7-(β-D-glucopyranosyloxy)-5-hydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX0788
CAS No.:1575672-58-0
- Yibeissine
Catalog No.:BCX0789
CAS No.:143502-51-6
- 6-hydroxyl kaempherol-3,6-O-diglucosyl-7-O-Glucuronic acid
Catalog No.:BCX0790
CAS No.:307950-53-4
- N-noratherosperminine
Catalog No.:BCX0791
CAS No.:74606-53-4
- Nor-rubrofusarin-6-O-β-D-gentiobioside
Catalog No.:BCX0792
CAS No.:245724-08-7
- 7,4′-Dihydroxyhomoisoflavane
Catalog No.:BCX0793
CAS No.:148462-00-4
- O-Cymen-5-ol
Catalog No.:BCX0794
CAS No.:39660-61-2
- Magnoloside B
Catalog No.:BCX0795
CAS No.:116872-05-0
Mining and functional characterization of NADPH-cytochrome P450 reductases of the DNJ biosynthetic pathway in mulberry leaves.[Pubmed:38395770]
BMC Plant Biol. 2024 Feb 23;24(1):133.
BACKGROUND: 1-Deoxynojirimycin (DNJ), the main active ingredient in mulberry leaves, with wide applications in the medicine and food industries due to its significant functions in lowering blood sugar, and lipids, and combating viral infections. Cytochrome P450 is a key enzyme for DNJ biosynthesis, its activity depends on the electron supply of NADPH-cytochrome P450 reductases (CPRs). However, the gene for MaCPRs in mulberry leaves remains unknown. RESULTS: In this study, we successfully cloned and functionally characterized two key genes, MaCPR1 and MaCPR2, based on the transcriptional profile of mulberry leaves. The MaCPR1 gene comprised 2064 bp, with its open reading frame (ORF) encoding 687 amino acids. The MaCPR2 gene comprised 2148 bp, and its ORF encoding 715 amino acids. The phylogenetic tree indicates that MaCPR1 and MaCPR2 belong to Class I and Class II, respectively. In vitro, we found that the recombinant enzymes MaCPR2 protein could reduce cytochrome c and ferricyanide using NADPH as an electron donor, while MaCPR1 did not. In yeast, heterologous co-expression indicates that MaCPR2 delivers electrons to MaC3'H hydroxylase, a key enzyme catalyzing the production of chlorogenic acid from 3-O-p-Coumaroylquinic acid. CONCLUSIONS: These findings highlight the orchestration of hydroxylation process mediated by MaCPR2 during the biosynthesis of secondary metabolite biosynthesis in mulberry leaves. These results provided a foundational understanding for fully elucidating the DNJ biosynthetic pathway within mulberry leaves.
BAHD acyltransferase induced by histone deacetylase inhibitor catalyzes 3-O-hydroxycinnamoylquinic acid formation in bamboo cells.[Pubmed:36305861]
Plant J. 2022 Dec;112(5):1266-1280.
Suspension-cultured cells of a bamboo species (Bambusa multiplex; Bm) produce 3-O-feruloylquinic acid (3-FQA) and 3-O-p-Coumaroylquinic acid (3-pCQA) by treatment with the histone deacetylase inhibitor suberoyl bis-hydroxamic acid (SBHA). Acyltransferases catalyzing the formation of 5-O-hydroxycinnamoylquinic acid esters by transesterification from hydroxycinnamoyl-CoAs to the C-5 hydroxy group of quinic acid (hydroxycinnamoyl-CoA:quinate hydroxycinnamoyltransferase, HQT) have been identified in the biosynthesis of chlorogenic acids and monolignols; however, an HQT that catalyzes the acylation of the C-3 hydroxy group of quinic acid has not been identified previously. In the present study, we purified a native HQT from SBHA-treated Bm cells. The purified enzyme preferentially accepted feruloyl-/p-coumaroyl-CoAs as acyl-donors and quinic acid as the acyl-acceptor, and the enzyme specifically formed 3-FQA and 3-pCQA but not 5-O-hydroxycinnamoylquinic acid esters or esters with shikimic acid. A cDNA (BmHQT1) encoding this HQT was isolated. Although BmHQT1 is a phylogenetically unique member of the BAHD acyltransferase superfamily that does not cluster with other HQTs, functional characterization of the recombinant enzyme verified that BmHQT1 catalyzes the regiospecific formation of 3-O-hydroxycinnamoylquinic acid esters. Transcript levels of BmHQT1 markedly increased in Bm cells cultured in the presence of SBHA. Moreover, elevated acetylation levels of histone H3 were observed in the coding region of BmHQT1 in the presence of SBHA, indicating that the induced accumulation of 3-FQA/3-pCQA by SBHA is caused by transcriptional activation of BmHQT1 by the action of SBHA as a histone deacetylase inhibitor. The results demonstrate the utility of HDAC inhibitors for discovery of cryptic secondary metabolites and unknown biosynthetic enzymes.
Phenolic compound profiles in Finnish apple (Malus x domestica Borkh.) juices and ciders fermented with Saccharomyces cerevisiae and Schizosaccharomyces pombe strains.[Pubmed:34749087]
Food Chem. 2022 Mar 30;373(Pt B):131437.
The phenolic compounds in juices and ciders made with Saccharomyces cerevisiae or Schizosaccharomyces pombe from eleven Finnish apple cultivars were analyzed using liquid chromatographic and mass spectrometric methods combined with multivariate data analysis. In general, the ciders contained less phenolic compounds than corresponding apple juices. In the studied apple juices and ciders, hydroxycinnamic acids were the most predominant, accounting for around 80% of total phenolic compounds. Apple juices contained more flavonol glycosides and dihydrochalcones whereas cider processing resulted in increased amount of free hydroxycinnamic acids. The contents of individual phenolic compounds were more dependent on the apple cultivars than the yeast species. Certain cultivars contained remarkably higher contents of dihydrochalcones and hydroxycinnamic acids when comparing with other cultivars. Ciders made using S. pombe remained higher contents of procyanidins and (+)-catechin while S. cerevisiae ciders contained higher individual hydroxycinnamic acids, such as 5-O-caffeoylquinic acid, 4-O-caffeoylquinic acid, 3-O-p-Coumaroylquinic acid, and 4-O-p-coumaroylquinic acid.
Activation of Cryptic Secondary Metabolite Biosynthesis in Bamboo Suspension Cells by a Histone Deacetylase Inhibitor.[Pubmed:34287751]
Appl Biochem Biotechnol. 2021 Nov;193(11):3496-3511.
Plants have evolved a diverse array of secondary metabolite biosynthetic pathways. Undifferentiated plant cells, however, tend to biosynthesize secondary metabolites to a lesser extent and sometimes not at all. This phenomenon in cultured cells is associated with the transcriptional suppression of biosynthetic genes due to epigenetic alterations, such as low histone acetylation levels and/or high DNA methylation levels. Here, using cultured cells of bamboo (Bambusa multiplex; Bm) as a model system, we investigated the effect of histone deacetylase (HDAC) inhibitors on the activation of cryptic secondary metabolite biosynthesis. The Bm suspension cells cultured in the presence of an HDAC inhibitor, suberoyl bis-hydroxamic acid (SBHA), exhibited strong biosynthesis of some compounds that are inherently present at very low levels in Bm cells. Two major compounds induced by SBHA were isolated and were identified as 3-O-p-Coumaroylquinic acid (1) and 3-O-feruloylquinic acid (2). Their productivities depended on the type of basal culture medium, initial cell density, and culture period, as well as the SBHA concentration. The biosynthesis of these two compounds was also induced by another HDAC inhibitor, trichostatin A. These results demonstrate the usefulness of HDAC inhibitors to activate cryptic secondary metabolite biosynthesis in cultured plant cells.
Tropical ulcer plant treatments used by Papua New Guinea's Apsokok nomads.[Pubmed:28478094]
J Ethnopharmacol. 2017 Jun 9;205:240-245.
ETHNOPHARMACOLOGICAL RELEVANCE: The tropical ulcer is a debilitating bacterial infection that is common in Papua New Guinea. Deploying healthcare infrastructure to remote and inaccessible rainforest locations is not practical, therefore local plants may be the best treatment option. Here we present an ethnobotanical survey of the tropical ulcer plant medicines used by the semi-nomadic Apsokok who roam the remote central mountains of Papua New Guinea's West New Britain Province. In vitro biological activity in assays relevant to tropical ulcer wound healing is also presented. MATERIALS AND METHODS: Focus groups and semi-structured interviews were used to acquire information on the uses of plants, vouchers of which were identified by comparison with authentic herbarium specimens. Antibacterial disc diffusion assays with Staphylococcus aureus and Fusobacterium ulcerans, MMP-9 enzyme inhibition and dermal fibroblast stimulation assays were carried out on plant saps and aqueous extracts of plant material. LC-MS was used to identify known plant metabolites. RESULTS: The ethnobotanical survey identified sixteen species that were used to treat tropical ulcers, all of which were applied topically. A subset of twelve species were investigated further in vitro. Four species produced zones of inhibition with S. aureus, all 12 species provided low level inhibition of MMP-9 and 8 species stimulated dermal fibroblast proliferation, although cytotoxicity occurred at higher concentrations. The extract of Homalium foetidum Benth. inhibited S. aureus and MMP-9 while at lower sub-cytotoxic concentrations stimulated fibroblast proliferation. Trans-3-O-p-Coumaroylquinic acid cis-3-O-p-Coumaroylquinic acid were detected in the aqueous extract of H. foetidum. CONCLUSIONS: Topical application of plant saps to wounds results in very high localised concentrations of plant metabolites which is likely to result in inhibition of MMP proteases. H. foetidum is a candidate plant for tropical ulcer treatment in remote areas.
Phenolic compound profiles and antioxidant capacity of Persea americana Mill. peels and seeds of two varieties.[Pubmed:22494370]
J Agric Food Chem. 2012 May 9;60(18):4613-9.
Avocado processing by the food and cosmetic industries yields a considerable amount of phenolic-rich byproduct such as peels and seeds. Utilization of these byproducts would be favorable from an economic point of view. Methanolic (80%) extracts obtained from lyophilized ground peels and seeds of avocado (Persea americana Mill.) of the Hass and Shepard varieties were characterized for their phenolic compound profiles using the HPLC-PAD technique. The structures of the identified compounds were subsequently unambiguously confirmed by ESI-MS. Compositional analysis revealed that the extracts contained four polyphenolic classes: flavanol monomers, proanthocyanidins, hydroxycinnamic acids, and flavonol glycosides. The presence of 3-O-caffeoylquinic acid, 3-O-p-Coumaroylquinic acid, and procyanidin A trimers was identified in seeds of both varieties. Intervarietal differences were apparent in the phenolic compound profiles of peels. Peels of the Shepard variety were devoid of (+)-catechin and procyanidin dimers, which were present in the peels of the Hass variety. Peels of both varieties contained 5-O-caffeoylquinic acid and quercetin derivatives. The differences in the phenolic profiles between varietals were also apparent in the different antioxidant activity of the extracts. The peel extracts had a higher total phenolic compound content and antioxidant activity when compared to the seed extracts. The highest TEAC and ORAC values were apparent in peels of the Haas variety in which they amounted to 0.16 and 0.47 mmol Trolox/g DW, respectively. No significant (p > 0.05) differences were apparent between the TEAC values of seeds of the two varieties but the ORAC values differed significantly (p < 0.05). Overall these findings indicate that both the seeds and peel of avocado can be utilized as a functional food ingredient or as an antioxidant additive.
[Studies on the chemical constituents in herbs of Hemistepta lyrata].[Pubmed:17048662]
Zhongguo Zhong Yao Za Zhi. 2006 May;31(10):812-3.
OBJECTIVE: To investigate the chemical constituents of Hemistepta lyrata. METHOD: The constituents of the EtOAc-soluble portions of the 95% ethanol extract were isolated and purified by means of chromatography. Compounds were identified by their physical characteristics and spectral features. RESULT: Five compounds were isolated and identified as caffeic acid (1), tracheloside (2), uracil (3), 8-carboxymethyl-p-hydroxycinnamic acid (4), and 3-O-p-Coumaroylquinic acid (5). CONCLUSION: Compounds 1-5 were isolated from this genus for the first time.


