ChrysotoxineCAS# 156951-82-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 156951-82-5 | SDF | Download SDF |
| PubChem ID | 5315860.0 | Appearance | Powder |
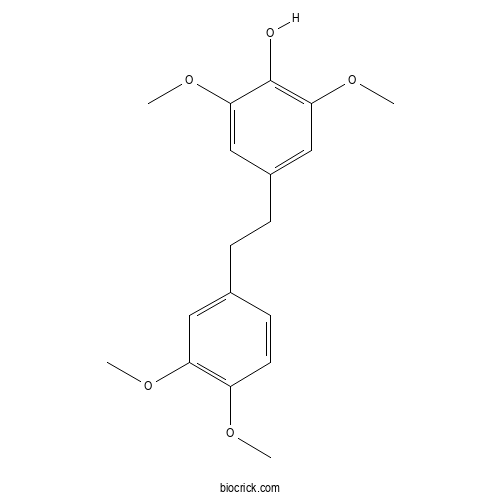
| Formula | C18H22O5 | M.Wt | 318.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[2-(3,4-dimethoxyphenyl)ethyl]-2,6-dimethoxyphenol | ||
| SMILES | COC1=C(C=C(C=C1)CCC2=CC(=C(C(=C2)OC)O)OC)OC | ||
| Standard InChIKey | YUHRVKGYFHPWRI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H22O5/c1-20-14-8-7-12(9-15(14)21-2)5-6-13-10-16(22-3)18(19)17(11-13)23-4/h7-11,19H,5-6H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Chrysotoxine Dilution Calculator

Chrysotoxine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1411 mL | 15.7055 mL | 31.411 mL | 62.822 mL | 78.5275 mL |
| 5 mM | 0.6282 mL | 3.1411 mL | 6.2822 mL | 12.5644 mL | 15.7055 mL |
| 10 mM | 0.3141 mL | 1.5705 mL | 3.1411 mL | 6.2822 mL | 7.8527 mL |
| 50 mM | 0.0628 mL | 0.3141 mL | 0.6282 mL | 1.2564 mL | 1.5705 mL |
| 100 mM | 0.0314 mL | 0.1571 mL | 0.3141 mL | 0.6282 mL | 0.7853 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Verbascose
Catalog No.:BCX0786
CAS No.:546-62-3
- Myricetin 3'-methyl-3-O-rutinoside
Catalog No.:BCX0785
CAS No.:55481-90-8
- Choerospondin
Catalog No.:BCX0784
CAS No.:81202-36-0
- 3-O-p-Coumaroylquinic acid
Catalog No.:BCX0783
CAS No.:5746-55-4
- Bixin
Catalog No.:BCX0782
CAS No.:6983-79-5
- Frangufoline
Catalog No.:BCX0781
CAS No.:19526-09-1
- Malonic acid
Catalog No.:BCX0780
CAS No.:90844-16-9
- Isogermafurenolide
Catalog No.:BCX0779
CAS No.:20267-89-4
- Nardoguaianone J
Catalog No.:BCX0778
CAS No.:443128-64-1
- Verrucosin
Catalog No.:BCX0777
CAS No.:83198-63-4
- Maltotriose
Catalog No.:BCX0776
CAS No.:1109-28-0
- 20-Deoxy,5-benzoyl-Ingenol
Catalog No.:BCX0775
CAS No.:54706-97-7
- 3-[[6-Deoxy-2-O-[6-O-[3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]-α-L-mannopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-7-(β-D-glucopyranosyloxy)-5-hydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX0788
CAS No.:1575672-58-0
- Yibeissine
Catalog No.:BCX0789
CAS No.:143502-51-6
- 6-hydroxyl kaempherol-3,6-O-diglucosyl-7-O-Glucuronic acid
Catalog No.:BCX0790
CAS No.:307950-53-4
- N-noratherosperminine
Catalog No.:BCX0791
CAS No.:74606-53-4
- Nor-rubrofusarin-6-O-β-D-gentiobioside
Catalog No.:BCX0792
CAS No.:245724-08-7
- 7,4′-Dihydroxyhomoisoflavane
Catalog No.:BCX0793
CAS No.:148462-00-4
- O-Cymen-5-ol
Catalog No.:BCX0794
CAS No.:39660-61-2
- Magnoloside B
Catalog No.:BCX0795
CAS No.:116872-05-0
- (-)-Epipodophyllotoxin
Catalog No.:BCX0796
CAS No.:4375-07-9
- Kanzonol D
Catalog No.:BCX0797
CAS No.:155233-20-8
- 17-Hydroxygracillin
Catalog No.:BCX0798
CAS No.:90308-85-3
- Pterin-6-carboxylic acid
Catalog No.:BCX0799
CAS No.:948-60-7
[Study on chemical bibenzyls in Dendrobium gratiosissimum].[Pubmed:33350266]
Zhongguo Zhong Yao Za Zhi. 2020 Oct;45(20):4929-4937.
Nineteen compounds were isolated and structurally characterized from an ethanol extract of Dendrobium gratiossimum, including dendrogratiol A(1), DDB-1(2), 3,4-dihydroxyl-5,3',4'-trimethoxybibenzyl(3), amoenylin(4), Chrysotoxine(5), DTB(6), 3,4,4'-trihydroxyl-5,3'-dimethoxybenzyl(7), 3-methylgiga(8), aloifol(9), gigantol tetramethyl ether(10), batatasin Ⅲ(11), moscatilin(12), moniliformine(13), gigantol(14), DMB(15), flavanthrinin(16), cannithrene-2(17), 3,4-dihydroxyl-5,4'-dimethoxystilbene(18) and 4-hydroxy-3,5,4'-trimethoxystilbene(19). 1 was a new compound, and 2-10, 16, 18 and 19 were obtained from this plant species for the first time. In vitro cytotoxic and antiviral activities of these isolates were evaluated, which displayed that 4 showed moderate cytotoxicity against human hepatoma cell line HepG2 with the IC_(50) of 10.15 mumol.L~(-1); 7 and 12 exhibited moderate inhibitory activity towards HIV virus with the IC_(50) of 9.35 and 9.15 mumol.L~(-1), respectively; and 10 displayed inhibitory activity against IAV virus with the IC_(50) of 8.90 mumol.L~(-1).
Immune modulatory effect of a novel 4,5-dihydroxy-3,3 ,4 -trimethoxybibenzyl from Dendrobium lindleyi.[Pubmed:32870935]
PLoS One. 2020 Sep 1;15(9):e0238509.
Dendrobium bibenzyls and phenanthrenes such as Chrysotoxine, cypripedin, gigantol and moscatilin have been reported to show promising inhibitory effects on lung cancer growth and metastasis in ex vivo human cell line models, suggesting their potential for clinical application in patients with lung cancer. However, it remains to be determined whether these therapeutic effects can be also seen in primary human cells and/or in vivo. In this study, we comparatively investigated the immune modulatory effects of bibenzyls and phenanthrenes, including a novel Dendrobium bibenzyl derivative, in primary human monocytes. All compounds were isolated and purified from a Thai orchid Dendrobium lindleyi Steud, a new source of therapeutic compounds with promising potential of tissue culture production. We detected increased frequencies of TNF- and IL-6-expressing monocytes after treatment with gigantol and cypripedin, whereas Chrysotoxine and moscatilin did not alter the expression of these cytokines in monocytes. Interestingly, the new 4,5-dihydroxy-3,3',4'-trimethoxybibenzyl derivative showed dose-dependent immune modulatory effects in lipopolysaccharide (LPS)-treated CD14lo and CD14hi monocytes. Together, our findings show immune modulatory effects of the new bibenzyl derivative from Dendrobium lindleyi on different monocyte sub-populations. However, therapeutic consequences of these different monocyte populations on human diseases including cancer remain to be investigated.
Recent research progress on natural small molecule bibenzyls and its derivatives in Dendrobium species.[Pubmed:32711292]
Eur J Med Chem. 2020 Oct 15;204:112530.
Orchidaceous plant Dendrobium genus is often used as a tonic, and its phenolic components have attracted attention for its anti-tumor and anti-diabetic complications. Bibenzyls is one of the essential phenolic active ingredients in the Dendrobium genus. At present, 89 bibenzyl derivatives have been extracted and identified from 46 Dendrobium species. The activity studies have shown that 42 compounds have pharmaceutical activity. Among them, 23 compounds showed antitumor activity; 7 compounds showed anti-diabetes and its complications activity; 10 compounds exhibited neuroprotective effects; 18 compounds showed antioxidant effects; 11 compounds had anti-inflammatory activity; 3 compounds had Antiplatelet aggregation effects; 3 compounds had antibacterial and antiviral effects. The Bibenzyls is small-molecular compounds of natural origin and widely sourced. Previous studies showed that the bibenzyls has good anti-tumor, anti-diabetes and its complications, and neuroprotective effects, and it has great potential for treating tumors, diabetes and its complications, Alzheimer's disease (AD) and Parkinson's disease (PD). Additionally, compounds such as moscatilin (1), gigantol (2) and Chrysotoxine (3) have been further studied as lead compounds, and compounds exhibited therapeutical effects had been synthesized. Enough pieces of evidences have shown that the Bibenzyls have good development prospects. This article reviews the pharmacological effects of bibenzyls in Dendrobium species and provides an idea for its further development.
Cancer Stem Cell-Suppressing Activity of Chrysotoxine, a Bibenzyl from Dendrobium pulchellum.[Pubmed:29217540]
J Pharmacol Exp Ther. 2018 Feb;364(2):332-346.
Cancer stem cells (CSCs) have been recognized as rare populations driving cancer progression, metastasis, and drug resistance in leading cancers. Attempts have been made toward identifying compounds that specifically target these CSCs. Therefore, investigations of novel therapeutic strategies for CSC targeting are required. The cytotoxic effects of Chrysotoxine on human non-small cell lung cancer-derived H460 and H23 cells were evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The effects of Chrysotoxine suppression of CSC-like phenotypes were determined in CSC-rich populations and primary CSCs in three-dimensional (3D) culture and in an extreme limiting dilution assay. Expression of CSC markers and associated proteins was determined by Western blot analyse and flow cytometry. We have reported herein the CSC-suppressing activity of Chrysotoxine, a bibenzyl compound isolated from Dendrobium pulchellum We have shown, to our knowledge for the first time, that Chrysotoxine dramatically suppresses CSC-like phenotypes of H460 and H23 cells. Treatment with Chrysotoxine significantly reduced the viability of 3D CSC-rich populations and concomitantly decreased known CSC markers. Chrysotoxine suppressed CSC phenotypes through downregulation of Src/protein kinase B (Akt) signaling. Active (phosphorylated Y416) Src was shown to regulate cancer stemness, since ectopic overexpression of Src strongly activated Akt and subsequently enhanced pluripotency transcription factor SRY (sex-determining region Y)-box 2 (Sox2)- mediating CSC phenotypes, whereas the short hairpin RNA of Src and an Src inhibitor (dasatinib) suppressed Akt, Sox2, and CSC properties. Importantly, Chrysotoxine was shown to suppress active Src/Akt signaling and in turn depleted Sox2-mediated CSCs. Our findings indicate a novel CSC-targeted role of Chrysotoxine and its regulation by Src/Akt and Sox2, which may be exploited for cancer treatment.
Determination of chrysotoxine in rat plasma by liquid chromatography-tandem mass spectrometry and its application to a rat pharmacokinetic study.[Pubmed:25069096]
J Chromatogr B Analyt Technol Biomed Life Sci. 2014 Sep 15;967:57-62.
Chrysotoxine (CTX), a naturally occurring bibenzyl compound isolated from Dendrobium species, has been reported to have neuroprotective effects. To evaluate its pharmacokinetics in rats, a rapid, sensitive and specific high performance liquid chromatography-tandem mass spectrometric (HPLC-MS/MS) method has been developed and validated for the quantification of CTX in rat plasma. Samples were pretreated using a simple liquid-liquid extraction with ethyl acetate and the chromatographic separation was performed on a C18 column with acetonitrile-water (90:10, v/v) as the mobile phase. CTX and the internal standard (wogonin) were detected using a tandem mass spectrometer in positive multiple reaction monitoring mode. Method validation revealed excellent linearity over the range 0.5-1000 ng/mL together with satisfactory intra- and inter-day precision, accuracy and recovery. Stability testing showed that CTX spiked into rat plasma was stable for 8 h at room temperature, for up to two weeks at -20 degrees C, and during three freeze-thaw cycles. Extracted samples were also observed to be stable over 24 h in an auto-sampler. The method was successfully used to investigate the pharmacokinetic profile of CTX after oral (100 mg/kg) and intravenous (25 mg/kg) administration in rats. CTX showed rapid excretion and low bioavailability in rats.
Anti-metastatic activities of bibenzyls from Dendrobium pulchellum.[Pubmed:23472473]
Nat Prod Commun. 2013 Jan;8(1):115-8.
Our investigation of the stem of Dendrobium pulchellum resulted in the isolation of four known bibenzyls, chrysotobibenzyl (1), Chrysotoxine (2), crepidatin (3) and moscatilin (4). The present study reveals for the first time the ability of these four compounds to facilitate anoikis and inhibit the growth of lung cancer cells in anchorage-independent condition. The preliminary data obtained disclose the inhibitory effect on cancer cell metastasis of the isolated compounds, and provide an important new approach for cancer drug development.
Chrysotoxine, a novel bibenzyl compound selectively antagonizes MPP(+), but not rotenone, neurotoxicity in dopaminergic SH-SY5Y cells.[Pubmed:22659498]
Neurosci Lett. 2012 Jul 11;521(1):76-81.
Chrysotoxine is a naturally occurring bibenzyl compound found in medicinal Dendrobium species. We previously reported that Chrysotoxine structure-specifically suppressed 6-hydroxydopamine (6-OHDA)-induced dopaminergic cell death. Whether Chrysotoxine and other structurally similar bibenzyl compounds could also inhibit the neurotoxicity of 1-methyl-4-phenyl pyridinium (MPP(+)) and rotenone has not been investigated. We showed herein that Chrysotoxine inhibited MPP(+), but not rotenone, induced dopaminergic cell death in SH-SY5Y cells. The overproduction of reactive oxygen species (ROS), mitochondrial dysfunction as indexed by the decrease in membrane potential, increase in calcium concentration and NF-kappaB activation triggered by MPP(+) were blocked by Chrysotoxine pretreatment. The imbalance between the pro-apoptotic signals (Bax, caspase-3, ERK and p38 MAPK) and the pro-survival signals (Akt/PI3K/GSK-3beta) induced by MPP(+) was partially or totally rectified by Chrysotoxine. The results indicated that ROS inhibition, mitochondria protection, NF-kappaB modulation and regulation of multiple signals determining cell survival and cell death were involved in the protective effects of Chrysotoxine against MPP(+) toxicity in SH-SY5Y cells. Given the different toxic profiles of 6-OHDA and MPP(+) as compared to rotenone, our results also indicated that DAT inhibition may partially account for the neuroprotective effects of Chrysotoxine.
Chrysotoxine, a novel bibenzyl compound, inhibits 6-hydroxydopamine induced apoptosis in SH-SY5Y cells via mitochondria protection and NF-kappaB modulation.[Pubmed:20708055]
Neurochem Int. 2010 Nov;57(6):676-89.
Some naturally occurring bibenzyl compounds have been reported as free radical scavengers. The present study tested our hypothesis that bibenzyl compounds may be neuroprotective against apoptosis induced by the neurotoxins. Five structurally similar bibenzyl derivatives were tested for their protective effect against 6-hydroxydopamine (6-OHDA) induced toxicity in the human neuroblastoma cell line SH-SY5Y. The results showed that one bibenzyl compound, namely Chrysotoxine, significantly attenuated 6-OHDA-induced cell death. The subsequent mechanism study demonstrated that Chrysotoxine significantly attenuated 6-OHDA-induced apoptosis characterized by DNA fragmentation and nuclear condensation in a dose-dependent manner. 6-OHDA-induced intracellular generation of reactive oxygen species (ROS), activation of p38 MAPK and ERK1/2, and mitochondrial dysfunctions, including the decrease of membrane potential, increase of intracellular free Ca2+, release of cytochrome c, imbalance of Bax/Bcl-2 ratio and activation of caspase-3 were strikingly attenuated by Chrysotoxine pretreatment. Meanwhile, Chrysotoxine counteracted NF-kappaB activation by blocking its translocation to the nucleus, thereby preventing up-regulation of inducible nitric oxide synthase (iNOS) and intracellular NO release. The data provide the first evidence that Chrysotoxine protects SH-SY5Y cells against 6-OHDA toxicity possibly through mitochondria protection and NF-kappaB modulation. Chrysotoxine is thus a candidate for further evaluation of its protection against neurodegeneration in Parkinson's disease.


