IsogermafurenolideCAS# 20267-89-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20267-89-4 | SDF | Download SDF |
| PubChem ID | 14038403.0 | Appearance | Powder |
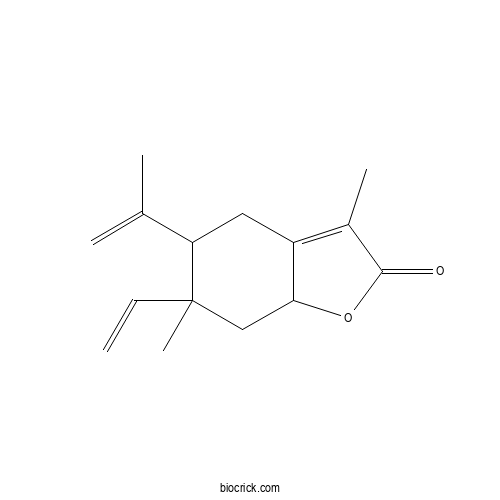
| Formula | C15H20O2 | M.Wt | 232.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 6-ethenyl-3,6-dimethyl-5-prop-1-en-2-yl-4,5,7,7a-tetrahydro-1-benzofuran-2-one | ||
| SMILES | CC1=C2CC(C(CC2OC1=O)(C)C=C)C(=C)C | ||
| Standard InChIKey | IEOHWPUTLCTSCQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H20O2/c1-6-15(5)8-13-11(7-12(15)9(2)3)10(4)14(16)17-13/h6,12-13H,1-2,7-8H2,3-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isogermafurenolide Dilution Calculator

Isogermafurenolide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3044 mL | 21.522 mL | 43.0441 mL | 86.0882 mL | 107.6102 mL |
| 5 mM | 0.8609 mL | 4.3044 mL | 8.6088 mL | 17.2176 mL | 21.522 mL |
| 10 mM | 0.4304 mL | 2.1522 mL | 4.3044 mL | 8.6088 mL | 10.761 mL |
| 50 mM | 0.0861 mL | 0.4304 mL | 0.8609 mL | 1.7218 mL | 2.1522 mL |
| 100 mM | 0.043 mL | 0.2152 mL | 0.4304 mL | 0.8609 mL | 1.0761 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nardoguaianone J
Catalog No.:BCX0778
CAS No.:443128-64-1
- Verrucosin
Catalog No.:BCX0777
CAS No.:83198-63-4
- Maltotriose
Catalog No.:BCX0776
CAS No.:1109-28-0
- 20-Deoxy,5-benzoyl-Ingenol
Catalog No.:BCX0775
CAS No.:54706-97-7
- 2,3-dihydroxypropyl 9-octadecenoate
Catalog No.:BCX0774
CAS No.:251983-54-7
- Nardoguaianone K
Catalog No.:BCX0773
CAS No.:443128-65-2
- 2-Hydroxycinnamicaldehyde
Catalog No.:BCX0772
CAS No.:3541-42-2
- (Z)-9-Nonadecene
Catalog No.:BCX0771
CAS No.:51865-02-2
- Hederoside D2
Catalog No.:BCX0770
CAS No.:20853-58-1
- Hirudonucleodisulfide B
Catalog No.:BCX0769
CAS No.:1072789-38-8
- 2α,6β,23-trihydroxyl oleanolic acid
Catalog No.:BCX0768
CAS No.:564-13-6
- L-Amygdalin
Catalog No.:BCX0767
CAS No.:29883-16-7
- Malonic acid
Catalog No.:BCX0780
CAS No.:90844-16-9
- Frangufoline
Catalog No.:BCX0781
CAS No.:19526-09-1
- Bixin
Catalog No.:BCX0782
CAS No.:6983-79-5
- 3-O-p-Coumaroylquinic acid
Catalog No.:BCX0783
CAS No.:5746-55-4
- Choerospondin
Catalog No.:BCX0784
CAS No.:81202-36-0
- Myricetin 3'-methyl-3-O-rutinoside
Catalog No.:BCX0785
CAS No.:55481-90-8
- Verbascose
Catalog No.:BCX0786
CAS No.:546-62-3
- Chrysotoxine
Catalog No.:BCX0787
CAS No.:156951-82-5
- 3-[[6-Deoxy-2-O-[6-O-[3-(4-hydroxyphenyl)-1-oxo-2-propen-1-yl]-β-D-glucopyranosyl]-α-L-mannopyranosyl]oxy]-2-(3,4-dihydroxyphenyl)-7-(β-D-glucopyranosyloxy)-5-hydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX0788
CAS No.:1575672-58-0
- Yibeissine
Catalog No.:BCX0789
CAS No.:143502-51-6
- 6-hydroxyl kaempherol-3,6-O-diglucosyl-7-O-Glucuronic acid
Catalog No.:BCX0790
CAS No.:307950-53-4
- N-noratherosperminine
Catalog No.:BCX0791
CAS No.:74606-53-4
Total synthesis, structural revision and biological evaluation of gamma-elemene-type sesquiterpenes.[Pubmed:30303229]
Org Biomol Chem. 2018 Oct 31;16(42):7843-7850.
Total synthesis and absolute configuration confirmation of gamma-elemene-type sesquiterpenes, which possess vast potential for biological activities, was investigated based on a convergent synthetic strategy. A key intermediate with all functional groups of this family of natural products was accessed by an intermolecular aldol reaction and then an acetylation of a known ketone (12) derived from commercially available verbenone. The versatile intermediate can be easily transformed into structurally different gamma-elemene-type sesquiterpenes based on control of base-promoted cyclization manipulation in different solvents. The utility of this robust approach is illustrated by the first syntheses of elema-1,3,7(11),8-tetraen-8,12-lactam (4') and 8beta-methoxy-Isogermafurenolide (6a), as well as the syntheses of elem-1,3,7,8-tetraen-8,12-olide (3) and hydroxyIsogermafurenolide (5) in only 6 or 7 steps. In addition, the structure of the reported 5betaH-elem-1,3,7,8-tetraen-8,12-olide (1) was revised as elem-1,3,7,8-tetraen-8,12-olide (3) by comparison of their identified datum, and the absolute configuration of elema-1,3,7(11),8-tetraen-8,12-lactam was confirmed as 4'. Furthermore, the inhibitory effect of all synthesized natural compounds and their natural analogues on cancer cell proliferation was evaluated. Among them compounds 3, 4 and 4' were found to possess potent inhibitory activity against Kasumi-1 and Pfeiffer. Meanwhile, preliminary structure-activity relationships for these compounds are discussed.
Inhibitory effect of sesquiterpene lactones and the sesquiterpene alcohol aromadendrane-4beta,10alpha-diol on memory impairment in a mouse model of Alzheimer.[Pubmed:26593432]
Eur J Pharmacol. 2015 Dec 15;769:195-202.
Alzheimer's disease (AD), a progressive neurodegenerative disorder of the aged brain with no known cause or cures, has become a major medical and social problem for industrialized countries. Cerebral deposition of amyloid-beta peptide (Abeta) is a critical feature of AD. The use of medicinal plants as an alternative form of prevention, or even as a possible treatment of AD, is therefore interesting areas of research. Sesquiterpene lactones and a sesquiterpene alcohol are compounds found in H. brasiliense that have several anti-oxidative and anti-inflammatory effects. In the present study, we investigated whether these compounds have neuroprotective effects in an amyloid-beta peptide-induced Alzheimer's disease mouse model. Mice were injected with Abeta1-42 peptide intracerebroventricularly and were subsequently injected (i.c.v.) with 1microg/site of IGM-A (15-acetoxy-Isogermafurenolide), IGM-H (15-hydroxy-Isogermafurenolide), PDA (Podoandin), EHP (1,2-epoxy-10alpha-hydroxy-podoandin), HDS (13-hydroxy-8,9-dehydroshizukanolide), and ARD (aromadendrane-4beta,10alpha-diol). Seven days after treatments the animals had their memory tested in the inhibitory avoidance. After the behavioral testing of animals the brains were removed and subjected to biochemical tests for oxidative stress. The results showed that ARD, HDS and PDA significantly ameliorated the Abeta1-42 peptide-induced memory impairment in the passive avoidance task (P<0.05). In addition, GSH activity was increased while the TBARS levels were decreased by treatment with these compounds. These results suggest that these compounds inhibit the cognitive deficit of animals induced peptide amyloid and may be potential candidates for Alzheimer's disease therapy.
Natural nitric oxide (NO) inhibitors from the rhizomes of Curcuma phaeocaulis.[Pubmed:26151445]
Org Biomol Chem. 2015 Aug 14;13(30):8349-58.
An exploration we carried out for isolating nitric oxide (NO) inhibitors from the rhizomes of Curcuma phaeocaulis afforded one new salvialane-type sesquiterpene, phasalvione (1), two novel nor-sesquiterpenes, phaeocaudione (2) and phaeocauone (3), one aromatic acid 3-methyl-4-(3-oxo-butyl)-benzoic acid (4), two gamma-elemene-type sesquiterpenes, 8beta(H)-elema-1,3,7(11)-trien-8,12-lactam (5) and 8beta-methoxy-Isogermafurenolide (6), one eudesmane-type sesquiterpene, phaeusmane I (7), and one cyclic diarylheptanoid, phaeoheptanoxide (8). Their structures were established based on extensive spectroscopic analysis. The absolute configurations of compounds 1 and 2 were assigned using the circular dichroism data of the [Rh2(OCOCF3)4] complex, and the absolute configuration of 1 was further established by single crystal X-ray crystallography. It is noteworthy that compounds 5-7 were racemates analyzed by chiral HPLC. Furthermore, the inhibitory effects of the isolated compounds on nitric oxide production in LPS-activated macrophages were evaluated. Compounds 1, 3 and 4 showed strong inhibitory activities on NO production with IC50 values of 7.46 +/- 0.69, 2.35 +/- 0.17 and 3.49 +/- 0.31 muM, respectively. A plausible biosynthetic pathway for 1-4 in C. phaeocaulis was also discussed.
Sesquiterpene lactones from the leaves of Hedyosmum brasiliense (Chloranthaceae).[Pubmed:23261032]
Phytochemistry. 2013 Mar;87:126-32.
Hedyosmum brasiliense Miq. is an endemic aromatic arborescent shrub that is the only representative of the Chloranthaceae in Brazil. There have been few studies seeking to determine its chemical constituents and/or pharmacological effects. This work describes the isolation and identification of sesquiterpene lactones from the leaves, including guaianolides, elemanolides and a lindenanolide. These were tested against Mycobacterium tuberculosis, together with podoandin, onoseriolide and some other common phenolics. The structures of the isolated compounds were determined based on extensive analysis of 1D and 2D NMR spectroscopic and MS data, as well as comparison with published data. The compounds found were the guaianolides, 1,2-epoxy-10alpha-hydroxy-podoandin and 1-hydroxy-10,15-methylenepodoandin, the elemenolide 15-acetoxy-Isogermafurenolide and the lindenanolide 8alpha/beta,9alpha-hydroxy-onoseriolide, along with the previously isolated guaianolide podoandin, the lindenanolide onoseriolide and the elemenolide 15-hydroxy-Isogermafurenolide. The phenolic compounds isolated were scopoletin, vanillin, vanillic acid, protocatechuic aldehyde and ethyl caffeate. The isolated sesquiterpene lactones did not show anti-mycobacterial activity against isoniazid-sensitive M. tuberculosis cultures at concentrations of 1-30 muM.
New cytotoxic terpenoids from the wood of Vepris punctata from the Madagascar Rainforest.[Pubmed:15165160]
J Nat Prod. 2004 May;67(5):895-8.
Continuation of the chemical examination of the cytotoxic constituents of the wood of Vepris punctata resulted in the isolation of the two new terpenoids 1 and 2 and eight known compounds, glechomanolide (3), Isogermafurenolide, (E,E)-germacra-1(10),4,7(11)-triene, alpha-amyrin, lupeol, lupeyl acetate, taraxerol, and 3-epi-taraxerol, in addition to the alkaloids reported reported previously. The structures of the two new compounds were established on the basis of 1D and 2D NMR spectroscopic data interpretation and chemical modifications. All the isolated compounds were tested against the A2780 human ovarian cancer cell line; the four sequiterpenoids showed moderate cytotoxic activity, while the six triterpenoids were inactive.


