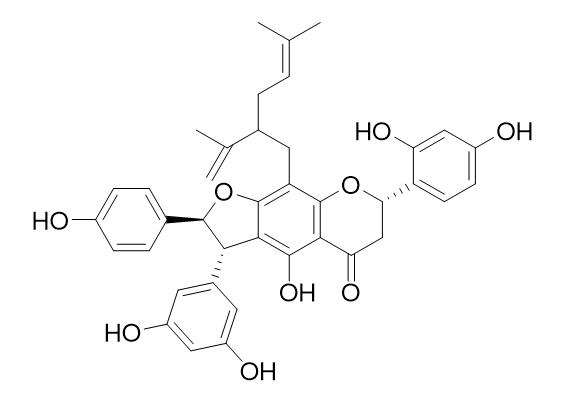
Alopecurone ACAS# 162558-89-6 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 162558-89-6 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C39H38O9 | M.Wt | 650.7 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Alopecurone A Dilution Calculator

Alopecurone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5368 mL | 7.684 mL | 15.3681 mL | 30.7361 mL | 38.4202 mL |
| 5 mM | 0.3074 mL | 1.5368 mL | 3.0736 mL | 6.1472 mL | 7.684 mL |
| 10 mM | 0.1537 mL | 0.7684 mL | 1.5368 mL | 3.0736 mL | 3.842 mL |
| 50 mM | 0.0307 mL | 0.1537 mL | 0.3074 mL | 0.6147 mL | 0.7684 mL |
| 100 mM | 0.0154 mL | 0.0768 mL | 0.1537 mL | 0.3074 mL | 0.3842 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Kidjolanin
Catalog No.:BCX0025
CAS No.:38395-01-6
- Hainanic acid B
Catalog No.:BCX0024
CAS No.:1637737-46-2
- 5-Hydroxy-4a,8-dimethyl-3-methylen-decahydroazuleno[6,5-b]furan-2(3H)-on
Catalog No.:BCX0023
CAS No.:114579-31-6
- Ganoderic acid GS-3
Catalog No.:BCX0022
CAS No.:1206781-66-9
- Sophoraflavone A
Catalog No.:BCX0021
CAS No.:105594-08-9
- 12beta-Acetoxy-3,7,11,15,23-pentaoxo-lanost-8,20-dien-26-oic acid
Catalog No.:BCX0020
CAS No.:1309931-91-6
- erythro-Austrobailignan-6
Catalog No.:BCX0019
CAS No.:114127-24-1
- Herbacetin 3-sophoroside-8-glucoside
Catalog No.:BCX0018
CAS No.:77298-68-1
- Regaloside I
Catalog No.:BCX0017
CAS No.:126239-78-9
- Dihydroconfertin
Catalog No.:BCX0016
CAS No.:68832-40-6
- Hosenkoside D
Catalog No.:BCX0015
CAS No.:156823-94-8
- 10-Deacetylcephalomannine
Catalog No.:BCX0014
CAS No.:76429-85-1
- Gossypetin 3-sophoroside-8-glucoside
Catalog No.:BCX0027
CAS No.:77306-93-5
- 5-O-Coumaroylquinic acid
Catalog No.:BCX0028
CAS No.:32451-86-8
- Catechin 7-O-beta-D-glucopyranoside
Catalog No.:BCX0029
CAS No.:65597-47-9
- Odontoside
Catalog No.:BCX0030
CAS No.:20300-50-9
- Caffeoylcalleryanin
Catalog No.:BCX0031
CAS No.:20300-49-6
- Ganolucidic acid E
Catalog No.:BCX0032
CAS No.:114567-50-9
- Apigenin-6-C-beta-D-glucopyranosyl-8-C-[alpha-L-rhamnopyranosyl-(1->2)]-beta-glucopyranoside
Catalog No.:BCX0033
CAS No.:1646598-06-2
- Tembetarine
Catalog No.:BCX0034
CAS No.:18446-73-6
- Scoparin
Catalog No.:BCX0035
CAS No.:301-16-6
- (Z)-Ferulic acid 4-O-beta-D-glucoside
Catalog No.:BCX0036
CAS No.:94942-20-8
- Methyl ganoderate A
Catalog No.:BCX0037
CAS No.:105742-78-7
- (3,4-Dihydroxyphenyl)methyl 3-(beta-D-glucopyranosyloxy)-4-hydroxybenzoate
Catalog No.:BCX0038
CAS No.:877461-90-0
High-Speed Counter-Current Chromatography with an Online Storage Technique for the Preparative Isolation and Purification of Dihydroflavonoids from Sophora alopecuroides L.[Pubmed:28589595]
Phytochem Anal. 2017 Nov;28(6):496-504.
INTRODUCTION: High-speed counter-current chromatography (HSCCC) is an efficient and non-absorption separation technique, but limitations still exist in simultaneous isolation of complex structures of natural products. Moreover, particular methods are various for different kinds of natural products. OBJECTIVE: A novel HSCCC strategy combined with an online storage recycling elution (OSR-CCC) technique was developed for the quick separation of naturally occurring dihydroflavonoids from the extract of the herb Sophora alopecuroides L. METHODOLOGY: In the separation procedure, a storage loop and two six-port valves were connected to a HSCCC system. Effluent A was subjected to an online storage loop and then to recycling separation three times after effluent B was collected in head-to-tail mode. After completion of the recycling separation of effluent A, the elution was switched to tail-to-head mode to collect effluent C. A biphasic solvent system of n-hexane/ethyl acetate/methanol/water (9:6:6:8, v/v/v/v) was used as the separation solvent during the whole elution. RESULTS: Six constituents were isolated simultaneously from the extract (200 mg) of S. alopecuroides by running HSCCC non-stop, and their purities were higher than 95.0%. Their structures were determined as the pterocarpan glycoside sophoratonkin (1) (10.0 mg) and five dihydroflavonoids, alopecurone F (2) (5.4 mg), lehmannin (3) (11.0 mg), Alopecurone A (4) (35.0 mg), sophoraflavanone G (5) (21.0 mg), alopecurone B (6) (31.0 mg). CONCLUSION: This recycling HSCCC method combined with an online storage technique could be a rapid, effective and simple approach to isolate stilbene-dihydroflavonoids from herbs of the Sophora genus simultaneously. Copyright (c) 2017 John Wiley & Sons, Ltd.
Dereplication of cytotoxic compounds from different parts of Sophora pachycarpa using an integrated method of HPLC, LC-MS and (1)H-NMR techniques.[Pubmed:27696895]
Nat Prod Res. 2017 Jun;31(11):1270-1276.
Sophora pachycarpa Schrenk ex C.A.Mey. is an annual plant belonging to the family Fabaceae. The cytotoxic activities of methanol-dichloromethane extracts (1:1) of different parts of S. pachycarpa were investigated on DU145 (prostate cancer cell line) and MCF-7 (breast cancer cell line) cell lines. The root extract of S. pachycarpa was the only extract that showed significant cytotoxic activity with IC50 values of 39.88 and 16.49 mug/mL on DU145 and MCF-7 cell lines, respectively. The root extract was then subjected to RP-HPLC for further fractionations. Among the isolated fractions from root extract, only one of them had remarkable cytotoxic effects with IC50 value of 26.43 on MCF-7 and 7.54 mug/mL on DU145 cell lines. Further purification led to isolation of a compound with IC50 values of 5.44 and 2.44 mug/mL on MCF-7 and DU145 cell lines, respectively. Based on (1)H NMR and (13)C NMR spectra, together with LC-MS, the structure of the purified compound was assigned as the flavonostilbene Alopecurone A.
Flavonostilbenes from Sophora alopecuroides L. as multidrug resistance associated protein 1 (MRP1) inhibitors.[Pubmed:24956120]
Nat Prod Res. 2014;28(23):2195-8.
Flavonoids have always attracted much attention due to their reversal activity on multidrug resistance (MDR). Eight flavonoids isolated from traditional Chinese medicine Sophora alopecuroides L. were applied to test their effect on MDR associated protein 1 (MRP1) through the established predicting assay. Three flavonostilbenes (Alopecurone A, B and D) were first found exhibiting potent inhibitory activity on MRP1. All of them dramatically increased 6-carboxyfluorescein diacetate and doxorubicin accumulation in MRP1-transfected U-2 OS cells. The compounds significantly increased the cytotoxicity and decreased the IC(5)(0) value of doxorubicin on the MDR cells (12-, 5- and 8-fold, respectively) at a non-toxic concentration (20 muM). Besides, Q-PCR analysis reveals that the MRP1 mRNA level in U-2 OS/MRP1 was also markedly decreased by the three compounds. These findings indicate a new therapeutic role of the herb. The three flavonostilbenes may have the possibility for further development as novel therapeutic reversal agents against MDR.
Antibacterial activity of flavanostilbenes against methicillin-resistant Staphylococcus aureus.[Pubmed:7576511]
Lett Appl Microbiol. 1995 Oct;21(4):219-22.
Three phytochemical compounds (Alopecurone A-C), flavanostilbenes which are produced by condensation between a hydroxyflavanone and a hydroxystilbene, were isolated as major components from the root of Sophora alopecuroides. They uniformly inhibited the growth of 21 strains of methicillin-resistant Staphylococcus aureus with minimum inhibitory concentrations of 3.13-6.25 micrograms ml-1.


