MaytansinolCAS# 57103-68-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57103-68-1 | SDF | Download SDF |
| PubChem ID | 156619867.0 | Appearance | Powder |
| Formula | C28H37ClN2O8 | M.Wt | 565.06 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
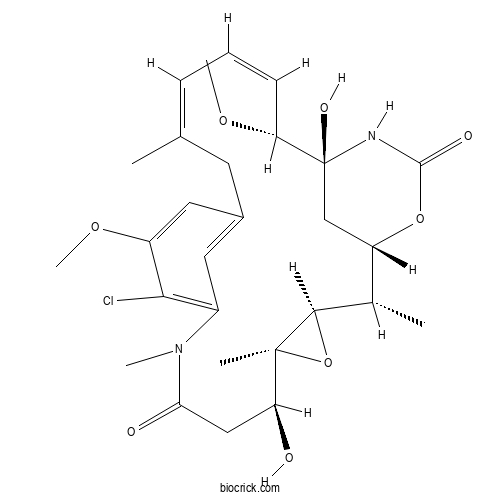
| Chemical Name | (1S,2S,3R,5S,6S,16Z,18Z,20R,21S)-11-chloro-6,21-dihydroxy-12,20-dimethoxy-2,5,9,16-tetramethyl-4,24-dioxa-9,22-diazatetracyclo[19.3.1.110,14.03,5]hexacosa-10,12,14(26),16,18-pentaene-8,23-dione | ||
| SMILES | CC1C2CC(C(C=CC=C(CC3=CC(=C(C(=C3)OC)Cl)N(C(=O)CC(C4(C1O4)C)O)C)C)OC)(NC(=O)O2)O | ||
| Standard InChIKey | QWPXBEHQFHACTK-FTDSKRPDSA-N | ||
| Standard InChI | InChI=1S/C28H37ClN2O8/c1-15-8-7-9-22(37-6)28(35)14-20(38-26(34)30-28)16(2)25-27(3,39-25)21(32)13-23(33)31(4)18-11-17(10-15)12-19(36-5)24(18)29/h7-9,11-12,16,20-22,25,32,35H,10,13-14H2,1-6H3,(H,30,34)/b9-7-,15-8-/t16-,20-,21-,22+,25+,27-,28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Maytansinol Dilution Calculator

Maytansinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7697 mL | 8.8486 mL | 17.6972 mL | 35.3945 mL | 44.2431 mL |
| 5 mM | 0.3539 mL | 1.7697 mL | 3.5394 mL | 7.0789 mL | 8.8486 mL |
| 10 mM | 0.177 mL | 0.8849 mL | 1.7697 mL | 3.5394 mL | 4.4243 mL |
| 50 mM | 0.0354 mL | 0.177 mL | 0.3539 mL | 0.7079 mL | 0.8849 mL |
| 100 mM | 0.0177 mL | 0.0885 mL | 0.177 mL | 0.3539 mL | 0.4424 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Maltooctaose
Catalog No.:BCX1111
CAS No.:66567-45-1
- Benzoyltropein
Catalog No.:BCX1110
CAS No.:19145-60-9
- Hyperforin acetate
Catalog No.:BCX1109
CAS No.:68324-06-1
- 4-(Acetyloxy)benzeneethanol
Catalog No.:BCX1108
CAS No.:60037-43-6
- Disodium uridine diphosphoglucose
Catalog No.:BCX1107
CAS No.:28053-08-9
- Notoginsenoside FP1
Catalog No.:BCX1106
CAS No.:1004988-73-1
- Notoginsenoside M
Catalog No.:BCX1105
CAS No.:394246-74-3
- Notoginsenoside N
Catalog No.:BCX1104
CAS No.:350586-56-0
- Gypenoside
Catalog No.:BCX1103
CAS No.:862286-45-1
- Gypenoside
Catalog No.:BCX1102
CAS No.:862286-47-3
- 20(R)-25-Methoxyprotopanaxadiol
Catalog No.:BCX1101
CAS No.:1050479-86-1
- 4-p-Coumaroylquinicacid
Catalog No.:BCX1100
CAS No.:1108200-72-1
- Irisolidone 7-O-glucoside
Catalog No.:BCX1113
CAS No.:126308-74-5
- Nifedipine impurity B
Catalog No.:BCX1114
CAS No.:50428-14-3
- Steviol-13-O-Glucoside
Catalog No.:BCX1115
CAS No.:60129-60-4
- Strigolactone
Catalog No.:BCX1116
CAS No.:76974-79-3
- Inflacoumarin A
Catalog No.:BCX1117
CAS No.:158446-33-4
- 22,23-Dihydroergosterol
Catalog No.:BCX1118
CAS No.:516-79-0
- Sphingomyelin
Catalog No.:BCX1119
CAS No.:6254-89-3
- Abyssinone II
Catalog No.:BCX1120
CAS No.:77263-08-2
- Orchioside B
Catalog No.:BCX1121
CAS No.:851780-22-8
- Beta-Tomatine
Catalog No.:BCX1122
CAS No.:17406-46-1
- Monensin B
Catalog No.:BCX1123
CAS No.:30485-16-6
- Jaligonic acid B
Catalog No.:BCX1124
CAS No.:2375176-78-4
Combinatorial Biosynthesis of 3-O-Carbamoylmaytansinol by Rational Engineering of the Tailoring Steps of Ansamitocins.[Pubmed:38377312]
ACS Synth Biol. 2024 Mar 15;13(3):721-727.
Currently, most maytansine-containing antibody-drug conjugates (ADCs) in clinical trials are prepared with DM1 or DM4, which in turn is synthesized mainly from ansamitocin P-3 (AP-3), a bacterial maytansinoid, isolated from Actinosynnema pretiosum. However, due to the high self-toxicity of AP-3 to A. pretiosum, the yield of AP-3 has been difficult to improve. Herein, a new maytansinoid with much lower self-toxicity to A. pretiosum, 3-O-carbamoylMaytansinol (CAM, 3), was designed and generated by introducing the 3-O-carbamoyltransferase gene asc21b together with the N-methyltransferase genes from exogenous maytansinoid gene clusters into the 3-O-acyltransferase gene (asm19) deleted mutant HGF052. Meanwhile, two new shunt products, 20-O-demethyl-19-dechloro-N-demethyl-4,5-desepoxy-CAM (4) and 20-O-demethyl-N-demethyl-4,5-desepoxy-CAM (5) were identified from the recombinant strain. Furthermore, by screening of liquid fermentation media, overexpression of bottleneck tailoring enzymes and the pathway-specific activator, the titer of CAM reached 498 mg/L in the engineered strain. Since the 3-O-carbamoyl group of CAM can be removed by chemical cleavage as AP-3 to produce Maytansinol, our work suggests that CAM may be a promising alternative to AP-3 in the future development of ADCs.
Maytansinol Functionalization: Towards Useful Probes for Studying Microtubule Dynamics.[Pubmed:36692211]
Chemistry. 2023 Jan 24;29(5):e202300069.
Invited for the cover of this issue are the groups of Professors Passarella and Pieraccini at the University of Milan, in collaboration with some of the members of TubInTrain consortium. The image depicts work with the elements of nature, in particular the destabilising effect of Maytansinol (the constellation) on microtubules (the trees). Read the full text of the article at 10.1002/chem.202203431.
Maytansinol Functionalization: Towards Useful Probes for Studying Microtubule Dynamics.[Pubmed:36468686]
Chemistry. 2023 Jan 24;29(5):e202203431.
Maytansinoids are a successful class of natural and semisynthetic tubulin binders, known for their potent cytotoxic activity. Their wider application as cytotoxins and chemical probes to study tubulin dynamics has been held back by the complexity of natural product chemistry. Here we report the synthesis of long-chain derivatives and maytansinoid conjugates. We confirmed that bulky substituents do not impact their high activity or the scaffold's binding mode. These encouraging results open new avenues for the design of new maytansine-based probes.
Maytansinol Derivatives: Side Reactions as a Chance for New Tubulin Binders.[Pubmed:34788896]
Chemistry. 2022 Jan 10;28(2):e202103520.
Maytansinol is a valuable precursor for the preparation of maytansine derivatives (known as maytansinoids). Inspired by the intriguing structure of the macrocycle and the success in targeted cancer therapy of the derivatives, we explored the Maytansinol acylation reaction. As a result, we were able to obtain a series of derivatives with novel modifications of the maytansine scaffold. We characterized these molecules by docking studies, by a comprehensive biochemical evaluation, and by determination of their crystal structures in complex with tubulin. The results shed further light on the intriguing chemical behavior of maytansinoids and confirm the relevance of this peculiar scaffold in the scenario of tubulin binders.
C3 ester side chain plays a pivotal role in the antitumor activity of Maytansinoids.[Pubmed:34144258]
Biochem Biophys Res Commun. 2021 Aug 20;566:197-203.
Maytansinoids, the chemical derivatives of Maytansine, are commonly used as potent cytotoxic payloads in antibody-drug conjugates (ADC). Structure-activity-relationship studies had identified the C3 ester side chain as a critical element for antitumor activity of maytansinoids. The maytansinoids bearing the methyl group at C3 position with D configuration were about 100 to 400-fold less cytotoxic than their corresponding L-epimers toward various cell lines. The detailed mechanism of how chirality affects the anticancer activity remains elusive. In this study, we determined the high-resolution crystal structure of tubulin in complex with Maytansinol, L-DM1-SMe and D-DM1-SMe. And we found the carbonyl oxygen atom of the ester moiety and the tail thiomethyl group at C3 side chain of L-DM1-SMe form strong intramolecular interaction with the hydroxyl at position 9 and the benzene ring, respectively, fixing the bioactive conformation and enhancing the binding affinity. Additionally, ligand-based and structure-based virtually screening methods were used to screen the commercially macrocyclic compounds library, and 15 macrocyclic structures were picketed out as putatively new maytansine-site inhibitors. Our study provides a possible strategy for the rational discovery of next-generation maytansine site inhibitors.
Characterization of in vivo biotransformations for trastuzumab emtansine by high-resolution accurate-mass mass spectrometry.[Pubmed:29958059]
MAbs. 2018 Oct;10(7):960-967.
Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate (ADC) designed for the treatment of HER2-positive cancers. T-DM1 is composed of the humanized monoclonal antibody trastuzumab connected to a maytansine derivative cytotoxic drug, via a nonreducible thioether linker at random lysine residues, and therefore has a very complex molecular structure. It was anticipated that T-DM1 undergoes biotransformations in circulation. However, there was limited knowledge on these structural changes due to bioanalytical challenges. Here, we have investigated the in vivo biotransformations of T-DM1 using a high-resolution accurate-mass (HR/AM) mass spectrometry approach. Three types of biotransformations were characterized for T-DM1 in circulation in tumor-bearing mice, including cysteine or glutathione adduct formation via maleimide exchange, loss of Maytansinol via ester hydrolysis, as well as addition of H(2)O via linker-drug hydrolysis. These results provide new insights into in vivo catabolism of T-DM1.
Constitutive overexpression of asm18 increases the production and diversity of maytansinoids in Actinosynnema pretiosum.[Pubmed:26572523]
Appl Microbiol Biotechnol. 2016 Mar;100(6):2641-9.
Ansamitocins isolated from Actinosynnema pretiosum, potent antitumor compounds, belong to the family of maytansinoids, and the antibody-maytansinoid conjugates are currently under different phases of clinical trials. The clinical applications of ansamitocins have stimulated extensive studies to improve their production yields. In this study, we investigated the function of a pathway-specific S treptomyces antibiotic regulatory protein (SARP) family regulator, Asm18, and observed that ectopic overexpression of the asm18 gene increased the production of N-demethyl-4,5-desepoxy-Maytansinol (2) to 50 mg/L in the HGF052 + pJTU824-asm18 strain, an increase by 4.7-fold compared to that of the control strain HGF052 + pJTU824. Real-time PCR analysis showed that the overexpression of the asm18 gene selectively increased the transcription levels of the genes involved in the biosynthesis of the starter unit (asm43), polyketide assembly (asmA), post-PKS modification (asm21), as well as the transcription levels of the regulatory gene (asm8), which is a specific LAL-type activator in ansamitocin biosynthesis. With the increase of fermentation titre, seven ansamitocin analogs (1-7) including three new ones (1, 5, and 6) and Maytansinol (7) were isolated from the HGF052 + pJTU824-asm18 strain. Our results not only pave the way for further improving the production of ansamitocin analogs but also indicate that the post-PKS modifications of ansamitocin biosynthesis are flexible, which brings a potential of producing Maytansinol, the most fascinating intermediate for the synthesis of antibody-maytansinoid conjugates, by optimizing the HGF052 and/or HGF052 + pJTU824-asm18 strains.
Trastuzumab-DM1: a clinical update of the novel antibody-drug conjugate for HER2-overexpressing breast cancer.[Pubmed:23196784]
Mol Med. 2013 Jan 22;18(1):1473-9.
Trastuzumab is a monoclonal antibody targeted against the HER2 tyrosine kinase receptor. Although trastuzumab is a very active agent in HER2-overexpressing breast cancer, the majority of patients with metastatic HER2-overexpressing breast cancer who initially respond to trastuzumab develop resistance within 1 year of initiation of treatment and, in the adjuvant setting, progress despite trastuzumab-based therapy. The antibody-drug conjugate trastuzumab-DM1 (T-DM1) was designed to combine the biological activity of trastuzumab with the targeted delivery of a highly potent antimicrotubule agent, DM1 (N-methyl-N-[3-mercapto-1-oxopropyl]-l-alanine ester of Maytansinol), a maytansine derivative, to HER2-overexpressing breast cancer cells. T-DM1 is the first antibody-drug conjugate with a nonreducible thioether linker in clinical trials. Phase I and II clinical trials of T-DM1 as a single agent and in combination with paclitaxel, docetaxel and pertuzumab have shown clinical activity and a favorable safety profile in patients with HER2-positive metastatic breast cancer. Two randomized phase III trials of T-DM1 are awaiting final results; the EMILIA trial is evaluating T-DM1 compared with lapatinib plus capecitabine, and early positive results have been reported. The MARIANNE trial is evaluating T-DM1 plus placebo versus T-DM1 plus pertuzumab versus trastuzumab plus a taxane. Here, we summarize evidence from clinical studies and discuss the potential clinical implications of T-DM1.
Two novel ansamitocin analogs from Actinosynnema pretiosum.[Pubmed:23061718]
Nat Prod Res. 2013;27(17):1532-6.
By using various column chromatography for purification, two new compounds of ansamitocin (1 and 2) were isolated from the extracts of fermentation broth of Actinosynnema pretiosum FIM06-0063. Their structures were established as Maytansinol-9-methyl ether-3-propionate (1) and Maytansinol-9-methyl ether-3-3'-methy-butyrate (2), respectively, by an extensive NMR analysis.
An isolable acyclic hemiacetal of ansamitocin P-3.[Pubmed:22374862]
Magn Reson Chem. 2012 Mar;50(3):256-9.
During impurity analysis of Maytansinol (2), produced from the reduction of ansamitocin P-3 (AP-3, 1), a surprisingly stable acyclic hemiacetal (4) was isolated. A combination of 1D and 2D NMR experiments, along with liquid chromatography-mass spectrometry data was used to confirm the structure. Comparison of NMR data to the previously reported bridged acetal (3), a by-product of AP-3 reduction, supports reassignment of the latter to the former. Additionally, ROESY data, in conjunction with minimum energy calculations, support intramolecular hydrogen bonding that is involved in stabilizing the hemiacetal. This report adds another example to the very short list of isolable acyclic hemiacetals.
Combinatorial effect of maytansinol and radiation in Drosophila and human cancer cells.[Pubmed:21504911]
Dis Model Mech. 2011 Jul;4(4):496-503.
Combination therapy, in which two or more agents are applied, is more effective than single therapies for combating cancer. For this reason, combinations of chemotherapy with radiation are being explored in clinical trials, albeit with an empirical approach. We developed a screen to identify, from the onset, molecules that act in vivo in conjunction with radiation, using Drosophila as a model. Screens through two small molecule libraries from the NCI Developmental Therapeutics Program yielded microtubule poisons; this class of agents is known to enhance the effect of radiation in mammalian cancer models. Here we report an analysis of one microtubule depolymerizing agent, Maytansinol isobutyrate (NSC292222; Maytansinol), in Drosophila and in human cancer cells. We find that the effect of Maytansinol is p53 dependent in Drosophila cells and human cancer cells, that Maytansinol enhances the effect of radiation in both systems, and that the combinatorial effect of drug and radiation is additive. We also uncover a differential sensitivity to Maytansinol between Drosophila cells and Drosophila larvae, which illustrates the value of studying cell behavior in the context of a whole organism. On the basis of these results, we propose that Drosophila might be a useful model for unbiased screens through new molecule libraries to find cancer drugs for combination therapy.
A new antitumour ansamitocin from Actinosynnema pretiosum.[Pubmed:20582809]
Nat Prod Res. 2010 Jul;24(12):1146-50.
A new compound of ansamitocin was isolated from the extracts of fermentation medium of mutant strain HGF052 derived from Actinosynnema pretiosum ssp. aurantium ATCC 31565, and identified as N-demethyl-desepoxy-9-methoxy-Maytansinol (1) on the basis of extensive spectroscopic methods. Bioassay results showed that compound 1 had cytotoxic activity against HL-60 and BEL-7402 cell lines.
Identification of asm19 as an acyltransferase attaching the biologically essential ester side chain of ansamitocins using N-desmethyl-4,5-desepoxymaytansinol, not maytansinol, as its substrate.[Pubmed:12047169]
J Am Chem Soc. 2002 Jun 12;124(23):6544-5.
The potent antitumor activity of the ansamitocins, polyketides isolated from Actinosynnema pretiosum, is absolutely dependent on a short acyl group esterified to the C-3 oxygen of the macrolactam ring. Asm19, a gene in the ansamitocin biosynthetic gene cluster with homology to macrolide O-acyltransferase genes, is thought to encode the enzyme catalyzing this esterification. A mutant carrying an inactivated asm19 no longer produced ansamitocins but accumulated N-desmethyl-4,5-desepoxyMaytansinol, rather than Maytansinol, indicating that the acylation is not the terminal step of the biosynthetic sequence. Bioconversion experiments and in vitro studies with recombinant Asm19, expressed in Escherichia coli, showed that the enzyme is very specific toward its alcohol substrate, converting N-desmethyl-4,5-desepoxyMaytansinol (but not Maytansinol) into ansamitocins, but rather promiscuous toward its acyl substrate, utilizing acetyl-, propionyl-, butyryl-, isobutyryl-, as well as isovaleryl-CoA.


