StrigolactoneCAS# 76974-79-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

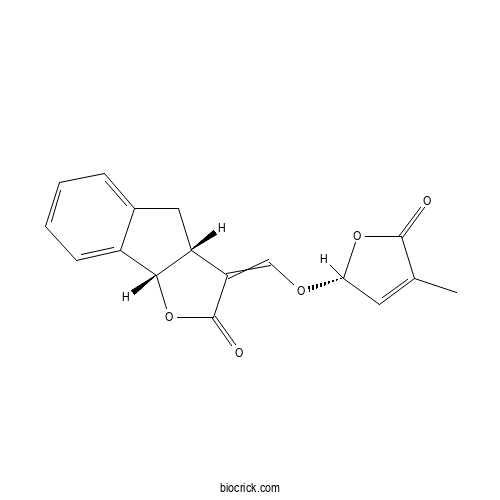
| Cas No. | 76974-79-3 | SDF | Download SDF |
| PubChem ID | 126963634.0 | Appearance | Powder |
| Formula | C17H14O5 | M.Wt | 298.29 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3aR,8bS)-3-[[(2R)-4-methyl-5-oxo-2H-furan-2-yl]oxymethylidene]-4,8b-dihydro-3aH-indeno[1,2-b]furan-2-one | ||
| SMILES | CC1=CC(OC1=O)OC=C2C3CC4=CC=CC=C4C3OC2=O | ||
| Standard InChIKey | XHSDUVBUZOUAOQ-BPLDGKMQSA-N | ||
| Standard InChI | InChI=1S/C17H14O5/c1-9-6-14(21-16(9)18)20-8-13-12-7-10-4-2-3-5-11(10)15(12)22-17(13)19/h2-6,8,12,14-15H,7H2,1H3/t12-,14-,15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Strigolactone Dilution Calculator

Strigolactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3524 mL | 16.7622 mL | 33.5244 mL | 67.0488 mL | 83.8111 mL |
| 5 mM | 0.6705 mL | 3.3524 mL | 6.7049 mL | 13.4098 mL | 16.7622 mL |
| 10 mM | 0.3352 mL | 1.6762 mL | 3.3524 mL | 6.7049 mL | 8.3811 mL |
| 50 mM | 0.067 mL | 0.3352 mL | 0.6705 mL | 1.341 mL | 1.6762 mL |
| 100 mM | 0.0335 mL | 0.1676 mL | 0.3352 mL | 0.6705 mL | 0.8381 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Steviol-13-O-Glucoside
Catalog No.:BCX1115
CAS No.:60129-60-4
- Nifedipine impurity B
Catalog No.:BCX1114
CAS No.:50428-14-3
- Irisolidone 7-O-glucoside
Catalog No.:BCX1113
CAS No.:126308-74-5
- Maytansinol
Catalog No.:BCX1112
CAS No.:57103-68-1
- Maltooctaose
Catalog No.:BCX1111
CAS No.:66567-45-1
- Benzoyltropein
Catalog No.:BCX1110
CAS No.:19145-60-9
- Hyperforin acetate
Catalog No.:BCX1109
CAS No.:68324-06-1
- 4-(Acetyloxy)benzeneethanol
Catalog No.:BCX1108
CAS No.:60037-43-6
- Disodium uridine diphosphoglucose
Catalog No.:BCX1107
CAS No.:28053-08-9
- Notoginsenoside FP1
Catalog No.:BCX1106
CAS No.:1004988-73-1
- Notoginsenoside M
Catalog No.:BCX1105
CAS No.:394246-74-3
- Notoginsenoside N
Catalog No.:BCX1104
CAS No.:350586-56-0
- Inflacoumarin A
Catalog No.:BCX1117
CAS No.:158446-33-4
- 22,23-Dihydroergosterol
Catalog No.:BCX1118
CAS No.:516-79-0
- Sphingomyelin
Catalog No.:BCX1119
CAS No.:6254-89-3
- Abyssinone II
Catalog No.:BCX1120
CAS No.:77263-08-2
- Orchioside B
Catalog No.:BCX1121
CAS No.:851780-22-8
- Beta-Tomatine
Catalog No.:BCX1122
CAS No.:17406-46-1
- Monensin B
Catalog No.:BCX1123
CAS No.:30485-16-6
- Jaligonic acid B
Catalog No.:BCX1124
CAS No.:2375176-78-4
- Zymosterol
Catalog No.:BCX1125
CAS No.:128-33-6
- (-)-Epitaxifolin
Catalog No.:BCX1126
CAS No.:114761-89-6
- 2,3,4,6-tetraacetate Salidroside
Catalog No.:BCX1127
CAS No.:28251-63-0
- Salidroside pentaacetate
Catalog No.:BCX1128
CAS No.:39032-08-1
Carotenoid Extraction from Plant Tissues.[Pubmed:38656505]
Methods Mol Biol. 2024;2788:3-18.
Carotenoids are the natural pigments available in nature and exhibit different colors such as yellow, red, and orange. These are a class of phytonutrients that have anti-cancer, anti-inflammatory, anti-oxidant, immune-modulatory, and anti-aging properties. These were used in food, pharmaceutical, nutraceutical, and cosmetic industries. They are divided into two classes: carotenes and xanthophylls. The carotenes are non-oxygenated derivatives and xanthophylls are oxygenated derivatives. The major source of carotenoids are vegetables, fruits, and tissues. Carotenoids also perform the roles of photoprotection and photosynthesis. In addition to the roles mentioned above, they are also involved and act as precursor molecules for the biosynthesis of phytohormones such as Strigolactone and abscisic acid. This chapter briefly introduces carotenoids and their extraction method from plant tissue. Proposed protocol describes the extraction of carotenoid using solvents chloroform and dichloromethane. Reverse-phase HPLC can be performed with C30 columns using gradient elution. The column C30 is preferred to the C18 column because the C30 column has salient features, which include selective nature in the separation of structural isomers and hydrophobic, long-chain compounds, and shows the best compatibility with highly aqueous mobile phases. A complete pipeline for the extraction of carotenoids from plant tissue is given in the present protocol.
Strigolactones affect the yield of Tartary buckwheat by regulating endogenous hormone levels.[Pubmed:38654155]
BMC Plant Biol. 2024 Apr 24;24(1):320.
BACKGROUND: As a newly class of endogenous phytohormones, Strigolactones (SLs) regulate crop growth and yield formation by interacting with other hormones. However, the physiological mechanism of SLs affect the yield by regulating the balance of endogenous hormones of Tartary buckwheat is still unclear. RESULTS: In this study, a 2-year field experiment was conducted on Tartary buckwheat (Jinqiao 2) to study the effects of different concentrations (0, 10, and 20 micromol/L) of artificial synthetic analogs of SLs (rac-GR24) and inhibitor of SL synthesis (Tis-108) on the growth, endogenous-hormone content, and yield of Tartary buckwheat. The main-stem branch number, grain number per plant, grain weight per plant, and yield of Tartary buckwheat continuously decreased with increased rac-GR24 concentration, whereas the main-stem diameter and plant height initially increased and then decreased. Rac-GR24 treatment significantly increased the content of SLs and abscisic acid (ABA) in grains, and it decreased the content of Zeatin (Z) + Zeatin nucleoside (ZR). Conversely, Tis-108 treatment decreased the content of SLs and ABA but increased the content of Z + ZR. Results of correlation analysis showed that the content of ABA and SLs, the ratio of SLs/(Z + ZR), SLs/ABA, and ABA/(Z + ZR) were significantly negatively correlated with the yield of Tartary buckwheat, and that Z + ZR content was significantly positively correlated with the yield. Regression analysis further showed that ABA/ (Z + ZR) can explain 58.4% of the variation in yield. CONCLUSIONS: In summary, by adjusting the level of endogenous SLs in Tartary buckwheat, the balance of endogenous hormones in grains can be changed, thereby exerting the effect on yield. The results can provide a new agronomic method for the high-yield cultivation of Tartary buckwheat.
Sequential activation of strigolactone and salicylate biosynthesis promotes leaf senescence.[Pubmed:38641854]
New Phytol. 2024 Apr 19.
Leaf senescence is a complex process strictly regulated by various external and endogenous factors. However, the key signaling pathway mediating leaf senescence remains unknown. Here, we show that Arabidopsis SPX1/2 negatively regulate leaf senescence genetically downstream of the Strigolactone (SL) pathway. We demonstrate that the SL receptor AtD14 and MAX2 mediate the age-dependent degradation of SPX1/2. Intriguingly, we uncover an age-dependent accumulation of SLs in leaves via transcriptional activation of SL biosynthetic genes by the transcription factors (TFs) SPL9/15. Furthermore, we reveal that SPX1/2 interact with the WRKY75 subclade TFs to inhibit their DNA-binding ability and thus repress transcriptional activation of salicylic acid (SA) biosynthetic gene SA Induction-Deficient 2, gating the age-dependent SA accumulation in leaves at the leaf senescence onset stage. Collectively, our new findings reveal a signaling pathway mediating sequential activation of SL and salicylate biosynthesis for the onset of leaf senescence in Arabidopsis.
Evaluation of granular formulated strigolactone analogs for Striga suicidal germination.[Pubmed:38634513]
Pest Manag Sci. 2024 Apr 18.
BACKGROUND: Striga hermonthica, an obligate root parasitic weed, poses significant threat to cereal production in sub-Saharan Africa. Lowering Striga seed bank in infested soils is a promising strategy to mitigate infestation levels. The dependency of Striga seed germination on Strigolactones opens up the possibility of a "suicidal germination" approach, where synthetic germination stimulants induce lethal germination in the absence of a host. Implementing this approach requires active germination stimulants with a suitable formulation for field application. Here, we describe the development of slow-releasing granular formulation of two potent germination stimulants 'Methyl Phenlactonoate 3' and 'Nijmegen-1' and the assessment of their activity under Lab, greenhouse, mini-field, and field conditions. RESULTS: Under laboratory conditions, the granular formulation of either of the two germination stimulants (1.25 mg per plate, corresponding to 0.09 mg a.i.) induced Striga seed germination at a rate of up to 43%. With 10 mg granular product (0.75 mg a.i.) per pot, we observed 77-83% reduction in Striga emergence under greenhouse pot conditions. Application of the formulated stimulants under artificially or naturally infested fields resulted in approximately 56%, 60%, and 72% reduction in Striga emergence in maize, sorghum, and millet fields in Kenya and Burkina Faso, respectively. CONCLUSION: Our findings on the newly designed granular formulation of Methyl Phenlactonoate 3 and Nijmegen-1 reveal encouraging prospects for addressing the Striga problem in Africa. These findings underscore several significant advantages of the formulated stimulants, including suitability for the African agricultural context, and, most importantly, their effectiveness in reducing Striga infection. This article is protected by copyright. All rights reserved.
DcERF109 regulates shoot branching by participating in strigolactone signal transduction in Dendrobium catenatum.[Pubmed:38618752]
Physiol Plant. 2024 Mar-Apr;176(2):e14286.
Shoot branching fundamentally influences plant architecture and agricultural yield. However, research on shoot branching in Dendrobium catenatum, an endangered medicinal plant in China, remains limited. In this study, we identified a transcription factor DcERF109 as a key player in shoot branching by regulating the expression of Strigolactone (SL) receptors DWARF 14 (D14)/ DECREASED APICAL DOMINANCE 2 (DAD2). The treatment of D. catenatum seedlings with GR24(rac)/TIS108 revealed that SL can significantly repress the shoot branching in D. catenatum. The expression of DcERF109 in multi-branched seedlings is significantly higher than that of single-branched seedlings. Ectopic expression in Arabidopsis thaliana demonstrated that overexpression of DcERF109 resulted in significant shoot branches increasing and dwarfing. Molecular and biochemical assays demonstrated that DcERF109 can directly bind to the promoters of AtD14 and DcDAD2.2 to inhibit their expression, thereby positively regulating shoot branching. Inhibition of DcERF109 by virus-induced gene silencing (VIGS) resulted in decreased shoot branching and improved DcDAD2.2 expression. Moreover, overexpression of DpERF109 in A. thaliana, the homologous gene of DcERF109 in Dendrobium primulinum, showed similar phenotypes to DcERF109 in shoot branch and plant height. Collectively, these findings shed new insights into the regulation of plant shoot branching and provide a theoretical basis for improving the yield of D. catenatum.
Sulfate Availability and Hormonal Signaling in the Coordination of Plant Growth and Development.[Pubmed:38612787]
Int J Mol Sci. 2024 Apr 3;25(7):3978.
Sulfur (S), one of the crucial macronutrients, plays a pivotal role in fundamental plant processes and the regulation of diverse metabolic pathways. Additionally, it has a major function in plant protection against adverse conditions by enhancing tolerance, often interacting with other molecules to counteract stresses. Despite its significance, a thorough comprehension of how plants regulate S nutrition and particularly the involvement of phytohormones in this process remains elusive. Phytohormone signaling pathways crosstalk to modulate growth and developmental programs in a multifactorial manner. Additionally, S availability regulates the growth and development of plants through molecular mechanisms intertwined with phytohormone signaling pathways. Conversely, many phytohormones influence or alter S metabolism within interconnected pathways. S metabolism is closely associated with phytohormones such as abscisic acid (ABA), auxin (AUX), brassinosteroids (BR), cytokinins (CK), ethylene (ET), gibberellic acid (GA), jasmonic acid (JA), salicylic acid (SA), and Strigolactones (SL). This review provides a summary of the research concerning the impact of phytohormones on S metabolism and, conversely, how S availability affects hormonal signaling. Although numerous molecular details are yet to be fully understood, several core signaling components have been identified at the crossroads of S and major phytohormonal pathways.
Design, Synthesis and Biological Evaluation of Novel Phenyl-Substituted Naphthoic Acid Ethyl Ester Derivatives as Strigolactone Receptor Inhibitor.[Pubmed:38612714]
Int J Mol Sci. 2024 Mar 31;25(7):3902.
Strigolactones (SLs) are plant hormones that regulate several key agronomic traits, including shoot branching, leaf senescence, and stress tolerance. The artificial regulation of SL biosynthesis and signaling has been considered as a potent strategy in regulating plant architecture and combatting the infection of parasitic weeds to help improve crop yield. DL1b is a previously reported SL receptor inhibitor molecule that significantly promotes shoot branching. Here, we synthesized 18 novel compounds based on the structure of DL1b. We performed rice tillering activity assay and selected a novel small molecule, C6, as a candidate SL receptor inhibitor. In vitro bioassays demonstrated that C6 possesses various regulatory functions as an SL inhibitor, including inhibiting germination of the root parasitic seeds Phelipanche aegyptiaca, delaying leaf senescence and promoting hypocotyl elongation of Arabidopsis. ITC analysis and molecular docking experiments further confirmed that C6 can interact with SL receptor proteins, thereby interfering with the binding of SL to its receptor. Therefore, C6 is considered a novel SL receptor inhibitor with potential applications in plant architecture control and prevention of root parasitic weed infestation.
Chemistry of Strigolactones, Key Players in Plant Communication.[Pubmed:38607659]
Chembiochem. 2024 Apr 12:e202400133.
Today, the use of artificial pesticides is questionable and the adaptation to global warming is a necessity. The promotion of favorable natural interactions in the rhizosphere offers interesting perspectives for changing the type of agriculture. Strigolactones (SLs), the latest class of phytohormones to be discovered, are also chemical mediators in the rhizosphere. We present in this review the diversity of natural SLs, their analogs, mimics, and probes essential for the biological studies of this class of compounds. Their biosynthesis and access by organic synthesis are highlighted especially concerning noncanonical SLs, the more recently discovered natural SLs. Organic synthesis of analogs, stable isotope-labeled standards, mimics, and probes are also reviewed here. In the last part, the knowledge about the SL perception is described as well as the different inhibitors of SL receptors that have been developed.
Design, Synthesis, and Bioactivities of N-Heterocyclic Ureas as Strigolactone Response Antagonists against Parasitic-Weed Seed Germination.[Pubmed:38593208]
J Agric Food Chem. 2024 Apr 9.
The pernicious parasitism exhibited by root parasitic weeds such as Orobanche and Striga poses substantial peril to agricultural productivity and global food security. This deleterious phenomenon hinges upon the targeted induction of the signaling molecule Strigolactones (SLs). Consequently, the identification of prospective SL antagonists holds significant promise in the realm of mitigating the infection of these pernicious weeds. In this study, we synthesized and characterized D12 based on a potent SL antagonist KK094. In vivo assay results demonstrated that D12 remarkably impedes the germination of Phelipanche aegyptiaca and Striga asiatica seeds, while also alleviating the inhibitory consequence of the SL analogue GR24 on hypocotyl elongation in Arabidopsis thaliana. The docking study and ITC assay indicated that D12 can interact strongly with the SL receptor protein, which may interfere with the binding of SL to the receptor protein as a result. In addition, the results of crop safety assessment tests showed that D12 had no adverse effects on rice seed germination and seedling growth and development. The outcomes obtained from the present study suggested that D12 exhibited promise as a prospective antagonist of SL receptors, thereby displaying substantial efficacy in impeding the seed germination process of root parasitic weeds, providing a promising basis for rational design and development of further Striga-specific herbicides.
Comparative Transcriptome Analysis Reveals Inhibitory Roles of Strigolactone in Axillary Bud Outgrowth in Ratoon Rice.[Pubmed:38592943]
Plants (Basel). 2024 Mar 21;13(6):899.
Axillary bud outgrowth, a key factor in ratoon rice yield formation, is regulated by several phytohormone signals. The regulatory mechanism of key genes underlying ratoon buds in response to phytohormones in ratoon rice has been less reported. In this study, GR24 (a Strigolactone analogue) was used to analyze the ratooning characteristics in rice cultivar Huanghuazhan (HHZ). Results show that the elongation of the axillary buds in the first seasonal rice was significantly inhibited and the ratoon rate was reduced at most by up to 40% with GR24 treatment. Compared with the control, a significant reduction in the content of auxin and cytokinin in the second bud from the upper spike could be detected after GR24 treatment, especially 3 days after treatment. Transcriptome analysis suggested that there were at least 742 and 2877 differentially expressed genes (DEGs) within 6 h of GR24 treatment and 12 h of GR24 treatment, respectively. Further bioinformatics analysis revealed that GR24 treatment had a significant effect on the homeostasis and signal transduction of cytokinin and auxin. It is noteworthy that the gene expression levels of OsCKX1, OsCKX2, OsGH3.6, and OsGH3.8, which are involved in cytokinin or auxin metabolism, were enhanced by the 12 h GR24 treatment. Taken overall, this study showed the gene regulatory network of auxin and cytokinin homeostasis to be regulated by Strigolactone in the axillary bud outgrowth of ratoon rice, which highlights the importance of these biological pathways in the regulation of axillary bud outgrowth in ratoon rice and would provide theoretical support for the molecular breeding of ratoon rice.
Removal of antibiotics by four microalgae-based systems for swine wastewater treatment under different phytohormone treatment.[Pubmed:38583677]
Bioresour Technol. 2024 Apr 5;400:130668.
This study examined the removal of typical antibiotics from simulated swine wastewater. Microalgae-bacteria/fungi symbioses were constructed using Chlorella ellipsoidea, endophytic bacteria (S395-2), and Clonostachys rosea as biomaterials. The growth, photosynthetic performance, and removal of three types of antibiotics (tetracyclines, sulfonamides, and quinolones) induced by four phytohormones were analyzed in each system. The results showed that all four phytohormones effectively improved the tolerance of symbiotic strains against antibiotic stress; Strigolactones (GR24) achieved the best performance. At 10(-9) M, GR24 achieved the best removal of antibiotics by C. elliptica + S395-2 + C. rosea symbiosis. The average removals of tetracycline, sulfonamide, and quinolone by this system reached 96.2-99.4 %, 75.2-81.1 %, and 66.8-69.9 %, respectively. The results of this study help to develop appropriate bio enhancement strategies as well as design and operate algal-bacterial-fungal symbiotic processes for the treatment of antibiotics-containing wastewater.
Genome-wide identification and in-silico expression analysis of CCO gene family in sunflower (Helianthus annnus) against abiotic stress.[Pubmed:38568355]
Plant Mol Biol. 2024 Apr 3;114(2):34.
Carotenoid cleavage oxygenases (CCOs) enzymes play an important role in plant growth and development by producing a wide array of apocarotenoids and their derivatives. These compounds are vital for colouring flowers and fruits and synthesizing plant hormones such as abscisic acid and Strigolactones. Despite their importance, the gene family responsible for CCO enzymes in sunflowers has not been identified. In this study, we identify the CCO genes of the sunflower plant to fill this knowledge gap. Phylogenetic and synteny analysis indicated that the Helianthus annnus CCO (HaCCO) genes were conserved in different plant species and they could be divided into three subgroups based on their conserved domains. Analysis using MEME tool and multiple sequence alignment identified conserved motifs in the HaCCO gene sequence. Cis-regulatory elements (CREs) analysis of the HaCCO genes indicated the presence of various responsive elements related to plant hormones, development, and responses to both biotic and abiotic stresses. This implies that these genes may respond to plant hormones, developmental cues, and drought stress, offering potential applications in the development of more resistant crops. Genes belonging to the 9-cis-epoxy carotenoid dioxygenases (NCED) subgroups predominantly exhibited chloroplast localization, whereas the genes found in other groups are primarily localized in the cytoplasm. These 21 identified HaCCOs were regulated by 60 miRNAs, indicating the crucial role of microRNAs in gene regulation in sunflowers. Gene expression analysis under drought stress revealed significant up-regulation of HaNCED16 and HaNCED19, genes that are pivotal in ABA hormone biosynthesis. During organ-specific gene expression analysis, HaCCD12 and HaCCD20 genes exhibit higher activity in leaves, indicating a potential role in leaf pigmentation. This study provides a foundation for future research on the regulation and functions of the CCO gene family in sunflower and beyond. There is potential for developing molecular markers that could be employed in breeding programs to create new sunflower lines resistant to biotic and abiotic stresses.
Strigolactones alleviate AlCl(3) stress by vacuolar compartmentalization and cell wall blocking in apple.[Pubmed:38565306]
Plant J. 2024 Apr 2.
Poor management and excess fertilization of apple (Malus domestica Borkh.) orchards are causing increasingly serious soil acidification, resulting in Al toxicity and direct poisoning of roots. Strigolactones (SLs) are reported to be involved in plant responses to abiotic stress, but their role and mechanism under AlCl(3) stress remain unknown. Here, we found that applying 1 mum GR24 (an SL analoge) significantly alleviated AlCl(3) stress of M26 apple rootstock, mainly by blocking the movement of Al through cell wall and by vacuolar compartmentalization of Al. RNA-seq analysis identified the core transcription factor gene MdWRKY53, and overexpressing MdWRKY53 enhanced AlCl(3) tolerance in transgenic apple plants through the same mechanism as GR24. Subsequently, we identified MdPMEI45 (encoding pectin methylesterase inhibitor) and MdALS3 (encoding an Al transporter) as downstream target genes of MdWRKY53 using chromatin immunoprecipitation followed by sequencing (ChIP-seq). GR24 enhanced the interaction between MdWRKY53 and the transcription factor MdTCP15, further increasing the binding of MdWRKY53 to the MdPMEI45 promoter and inducing MdPMEI45 expression to prevent Al from crossing cell wall. MdWRKY53 also bound to the promoter of MdALS3 and enhanced its transcription to compartmentalize Al in vacuoles under AlCl(3) stress. We therefore identified two modules involved in alleviating AlCl(3) stress in woody plant apple: the SL-WRKY+TCP-PMEI module required for excluding external Al by blocking the entry of Al(3+) into cells and the SL-WRKY-ALS module allowing internal detoxification of Al through vacuolar compartmentalization. These findings lay a foundation for the practical application of SLs in agriculture.
Strigolactones Might Regulate Ovule Development after Fertilization in Xanthoceras sorbifolium.[Pubmed:38542248]
Int J Mol Sci. 2024 Mar 14;25(6):3276.
Strigolactones (SLs) were recently defined as a novel class of plant hormones that act as key regulators of diverse developmental processes and environmental responses. Much research has focused on SL biosynthesis and signaling in roots and shoots, but little is known about whether SLs are produced in early developing seeds and about their roles in ovule development after fertilization. This study revealed that the fertilized ovules and early developing pericarp in Xanthoceras sorbifolium produced minute amounts of two Strigolactones: 5-deoxystrigol and strigol. Their content decreased in the plants with the addition of exogenous phosphate (Pi) compared to those without the Pi treatment. The exogenous application of an SL analog (GR24) and a specific inhibitor of SL biosynthesis (TIS108) affected early seed development and fruit set. In the Xanthoceras genome, we identified 69 potential homologs of genes involved in SL biological synthesis and signaling. Using RNA-seq to characterize the expression of these genes in the fertilized ovules, 37 genes were found to express differently in the fertilized ovules that were aborting compared to the normally developing ovules. A transcriptome analysis also revealed that in normally developing ovules after fertilization, 12 potential invertase genes were actively expressed. Hexoses (glucose and fructose) accumulated at high concentrations in normally developing ovules during syncytial endosperm development. In contrast, a low ratio of hexose and sucrose levels was detected in aborting ovules with a high Strigolactone content. XsD14 virus-induced gene silencing (VIGS) increased the hexose content in fertilized ovules and induced the proliferation of endosperm free nuclei, thereby promoting early seed development and fruit set. We propose that the crosstalk between sugar and Strigolactone signals may be an important part of a system that accurately regulates the abortion of ovules after fertilization. This study is useful for understanding the mechanisms underlying ovule abortion, which will serve as a guide for genetic or chemical approaches to promote seed yield in Xanthoceras.
An ecotoxicological assessment of a strigolactone mimic used as the active ingredient in a plant biostimulant formulation.[Pubmed:38537480]
Ecotoxicol Environ Saf. 2024 Apr 15;275:116244.
A risk assessment on the aquatic toxicity of the plant biostimulant Strigolactone mimic (2-(4-methyl-5-oxo-2,5-dihydro-furan-2-yloxy)-benzo[de]isoquinoline-1,3-dione (SL-6) was performed using a suite of standardised bioassays representing different trophic groups and acute and chronic endpoints. In freshwater, three trophic groups of algae, crustacea and fish were used. Whilst in seawater, algae (unicellular and macroalgae), Crustacea and Mollusca were employed. In addition, the genotoxicity of SL-6 was determined with the comet assessment performed on unicellular marine algae, oysters, and fish embryos. This was the first time ecotoxicity tests have been performed on SL-6. In freshwater, the lowest LOEC was measured in the unicellular algae at 0.31 mg/L SL-6. Although, similar LOEC values were found for embryo malformations and impacts on hatching rate in zebrafish (LOEC 0.31-0.33 mg/L). Consistent malformations of pericardial and yolk sac oedemas were identified in the zebrafish embryos at 0.31 mg/L. In marine species, the lowest LOEC was found for both Tisbe battagliai mortality and microalgae growth at an SL-6 concentration of 1.0 mg/L. Significant genotoxicity was observed above control levels at 0.0031 mg/L SL-6 in the unicellular algae and 0.001 mg/L SL-6 in the oyster and zebrafish larvae. When applying the simple risk assessment, based on the lowest NOECs and appropriate assessment factors, the calculated predicted no effect concentration (PNEC), for the ecotoxicity and the genotoxicity tests were 1.0 microg/L and 0.01 microg/L respectively.


