3-Nitro-L-tyrosineCAS# 621-44-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 621-44-3 | SDF | Download SDF |
| PubChem ID | 65124 | Appearance | Colorless cryst. |
| Formula | C9H10N2O5 | M.Wt | 226.19 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
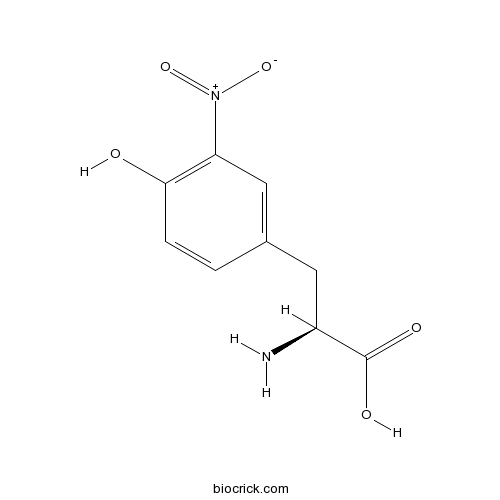
| SMILES | C1=CC(=C(C=C1CC(C(=O)O)N)[N+](=O)[O-])O | ||
| Standard InChIKey | FBTSQILOGYXGMD-LURJTMIESA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3-Nitro-L-tyrosine,an oxidative stress marker associated with neurodegenerative disorders.3-Nitro-L-tyrosine could as solid-phase extraction (SPE) sorbent material. |
| Structure Identification | Biosens Bioelectron. 2013 Feb 15;40(1):336-41.Developments in the synthesis of a water compatible molecularly imprinted polymer as artificial receptor for detection of 3-nitro-L-tyrosine in neurological diseases.[Pubmed: 22922080]A highly selective water compatible molecularly imprinted polymer (MIP) for 3-Nitro-L-tyrosine (3NT), an oxidative stress marker associated with neurodegenerative disorders, was prepared and its use as solid-phase extraction (SPE) sorbent material was demonstrated. The MIP was prepared by bulk polymerization using methacrylic acid as functional monomer and acetonitrile as porogen with traces of acetic acid and trifluoroacetic acid. |

3-Nitro-L-tyrosine Dilution Calculator

3-Nitro-L-tyrosine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4211 mL | 22.1053 mL | 44.2106 mL | 88.4212 mL | 110.5265 mL |
| 5 mM | 0.8842 mL | 4.4211 mL | 8.8421 mL | 17.6842 mL | 22.1053 mL |
| 10 mM | 0.4421 mL | 2.2105 mL | 4.4211 mL | 8.8421 mL | 11.0527 mL |
| 50 mM | 0.0884 mL | 0.4421 mL | 0.8842 mL | 1.7684 mL | 2.2105 mL |
| 100 mM | 0.0442 mL | 0.2211 mL | 0.4421 mL | 0.8842 mL | 1.1053 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Tyr(3-NO2)-OH
- Heliotrine N-oxide
Catalog No.:BCN1983
CAS No.:6209-65-0
- Nudiposide
Catalog No.:BCN7437
CAS No.:62058-46-2
- Hydroxycitric acid
Catalog No.:BCN2912
CAS No.:6205-14-7
- Onitisin 2'-O-glucoside
Catalog No.:BCN4150
CAS No.:62043-53-2
- 4-(Dimethylamino)cinnamaldehyde
Catalog No.:BCN4968
CAS No.:6203-18-5
- Ginsenoside F3
Catalog No.:BCN1077
CAS No.:62025-50-7
- Ginsenoside F2
Catalog No.:BCN1245
CAS No.:62025-49-4
- Juncusol
Catalog No.:BCN2926
CAS No.:62023-90-9
- Cyclobenzaprine HCl
Catalog No.:BCC6496
CAS No.:6202-23-9
- Bulgarsenine
Catalog No.:BCN2065
CAS No.:62018-77-3
- Helichrysetin
Catalog No.:BCN4149
CAS No.:62014-87-3
- p-Menthane-1,2,8-triol
Catalog No.:BCN4148
CAS No.:62014-81-7
- Isovanillin
Catalog No.:BCN2502
CAS No.:621-59-0
- Scutebarbatine E
Catalog No.:BCN8396
CAS No.:910099-77-3
- Benzyl carbamate
Catalog No.:BCC8871
CAS No.:621-84-1
- Rauwolscine hydrochloride
Catalog No.:BCC6834
CAS No.:6211-32-1
- Boc-Phe(4-Br)-OH
Catalog No.:BCC3159
CAS No.:62129-39-9
- Boc-Phe(4-I)-OH
Catalog No.:BCC3260
CAS No.:62129-44-6
- N-Methylcyclohexaneethaneamine
Catalog No.:BCN1795
CAS No.:62141-38-2
- Z-Prolinol
Catalog No.:BCC2709
CAS No.:6216-63-3
- (-)-Syringaresinol
Catalog No.:BCN3417
CAS No.:6216-81-5
- Mirandin B
Catalog No.:BCN6581
CAS No.:62163-24-0
- Benzyl L-(+)-mandelate
Catalog No.:BCC8873
CAS No.:62173-99-3
- 1,2-Bis(phenylthio)ethane
Catalog No.:BCC8415
CAS No.:622-20-8
Developments in the synthesis of a water compatible molecularly imprinted polymer as artificial receptor for detection of 3-nitro-L-tyrosine in neurological diseases.[Pubmed:22922080]
Biosens Bioelectron. 2013 Feb 15;40(1):336-41.
A highly selective water compatible molecularly imprinted polymer (MIP) for 3-Nitro-L-tyrosine (3NT), an oxidative stress marker associated with neurodegenerative disorders, was prepared and its use as solid-phase extraction (SPE) sorbent material was demonstrated. The MIP was prepared by bulk polymerization using methacrylic acid as functional monomer and acetonitrile as porogen with traces of acetic acid and trifluoroacetic acid. In order to evaluate its binding properties, the MIP was analyzed by batch rebinding experiments and subsequently used as SPE sorbent for the selective clean-up and pre-concentration of 3NT from standard solutions and spiked human urine samples. The results obtained from batch rebinding experiments showed the presence of two association constants corresponding to high-affinity (Ka 4.20x10(3) M(-1)) and low-affinity (Ka 0.79x10(3) M(-1)) binding sites. Standard mixture solution loaded on MIP-SPE cartridge gave a recovery of 95% for 3NT, while the other compounds were totally eluted during washing step. Percentage of recovery higher than 90%, with relative standard deviation of 2%, was also obtained when a maximum of 55 mug of 3NT is used in spiked urine sample and loaded into the cartridge. Validation of the analytical method for 3NT quantification in human urine gave 0.7 mug mL(-1) of limit of detection, a linear range of 2.5-55 mug mL(-1) with a relative standard deviation of 2%.


