4-Hydroxyalternariol 9-methyl etherCAS# 959417-17-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 959417-17-5 | SDF | Download SDF |
| PubChem ID | 24899916 | Appearance | Powder |
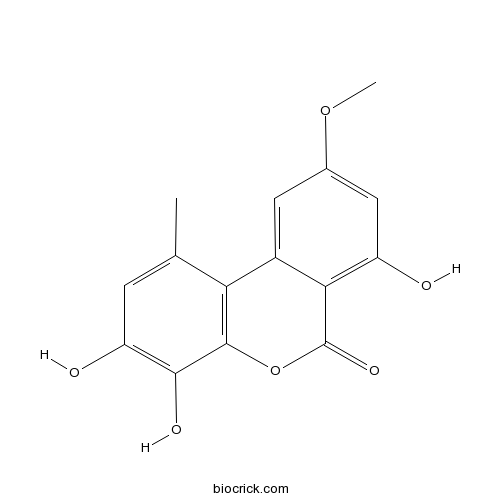
| Formula | C15H12O6 | M.Wt | 288.25 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,4,7-trihydroxy-9-methoxy-1-methylbenzo[c]chromen-6-one | ||
| SMILES | CC1=CC(=C(C2=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O)O | ||
| Standard InChIKey | BVYAURIYXKOUPX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12O6/c1-6-3-10(17)13(18)14-11(6)8-4-7(20-2)5-9(16)12(8)15(19)21-14/h3-5,16-18H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 4-Hydroxyalternariol 9-methyl ether shows antibacterial activity against several tested bacterial pathogens with minimum inhibitory concentration values in the range of 86.7-364.7 uM. 2. 4-Hydroxyalternariol 9-methyl ether displays promising antioxidant effects. |
| Targets | Antifection |

4-Hydroxyalternariol 9-methyl ether Dilution Calculator

4-Hydroxyalternariol 9-methyl ether Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4692 mL | 17.3461 mL | 34.6921 mL | 69.3842 mL | 86.7303 mL |
| 5 mM | 0.6938 mL | 3.4692 mL | 6.9384 mL | 13.8768 mL | 17.3461 mL |
| 10 mM | 0.3469 mL | 1.7346 mL | 3.4692 mL | 6.9384 mL | 8.673 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6938 mL | 1.3877 mL | 1.7346 mL |
| 100 mM | 0.0347 mL | 0.1735 mL | 0.3469 mL | 0.6938 mL | 0.8673 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PF 750
Catalog No.:BCC7641
CAS No.:959151-50-9
- A922500
Catalog No.:BCC2333
CAS No.:959122-11-3
- 2-Benzoylacetanilide
Catalog No.:BCC8560
CAS No.:959-66-0
- Sotalol hydrochloride
Catalog No.:BCC5165
CAS No.:959-24-0
- 12-Hydroxy-8(17),13-labdadien-16,15-olide
Catalog No.:BCN1298
CAS No.:958885-86-4
- GSK1059615
Catalog No.:BCC4984
CAS No.:958852-01-2
- SID 26681509
Catalog No.:BCC2362
CAS No.:958772-66-2
- (E)-1-(4-Hydroxyphenyl)dec-1-en-3-one
Catalog No.:BCN4031
CAS No.:958631-84-0
- Momordin IIa
Catalog No.:BCN3474
CAS No.:95851-50-6
- Momordin II
Catalog No.:BCN3473
CAS No.:95851-41-5
- Ipsapirone
Catalog No.:BCC7201
CAS No.:95847-70-4
- Hedyotisol B
Catalog No.:BCN4752
CAS No.:95839-45-5
- 5,7,4-Trihydroxy-3,6-dimethoxy-3-prenylflavone
Catalog No.:BCN1297
CAS No.:959421-20-6
- TC-E 5005
Catalog No.:BCC6227
CAS No.:959705-64-7
- Guggulsterone Z
Catalog No.:BCN3793
CAS No.:95975-55-6
- CH5138303
Catalog No.:BCC5364
CAS No.:959763-06-5
- Guajadial
Catalog No.:BCN4509
CAS No.:959860-49-2
- Aminothiazole
Catalog No.:BCC4623
CAS No.:96-50-4
- Mepivacaine
Catalog No.:BCC9020
CAS No.:96-88-8
- 2-hexyl-4-Pentynoic Acid
Catalog No.:BCC6480
CAS No.:96017-59-3
- SD 1008
Catalog No.:BCC2442
CAS No.:960201-81-4
- Vortioxetine (Lu AA21004) HBr
Catalog No.:BCC1213
CAS No.:960203-27-4
- Meropenem
Catalog No.:BCC2489
CAS No.:96036-03-2
- ONX-0914 (PR-957)
Catalog No.:BCC2095
CAS No.:960374-59-8
Dibenzo-alpha-pyrones from the endophytic fungus Alternaria sp. Samif01: isolation, structure elucidation, and their antibacterial and antioxidant activities.[Pubmed:27388053]
Nat Prod Res. 2017 Feb;31(4):387-396.
The EtOAc extract of the liquid fermentation of Alternaria sp. Samif01, an endophytic fungus obtained from Salvia miltiorrhiza Bunge, showed antibacterial activity against several tested bacterial pathogens. Fractionation of this extract led to the isolation of seven dibenzo-alpha-pyrones (1-7), including one new compound, 2-acetoxy-2-epi-altenuene (1) and one new natural product, 3-epi-dihydroaltenuene A (2). The structures of the new metabolites were elucidated by comprehensive analysis of the spectroscopic data including (1D, 2D) NMR and HRESIMS, while the absolute configuration of 1 was determined by TDDFT-ECD computation. Altenuisol (5), 4-hydroxyalternariol-9-methyl ether (6), and alternariol (7) showed inhibitory activities against the tested bacteria with minimum inhibitory concentration values in the range of 86.7-364.7 muM. A preliminary structure-antibacterial activity relationship was discussed. In addition, compounds 2, 5 and 6 displayed promising antioxidant effects using DPPH and hydroxyl radical assays. The cytotoxicity of the isolated compounds was evaluated as well.


