TC-E 5005Potent and selective PDE10A inhibitor CAS# 959705-64-7 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 959705-64-7 | SDF | Download SDF |
| PubChem ID | 24802717 | Appearance | Powder |
| Formula | C15H18N4O | M.Wt | 270.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 1eq. HCl and to 100 mM in ethanol | ||
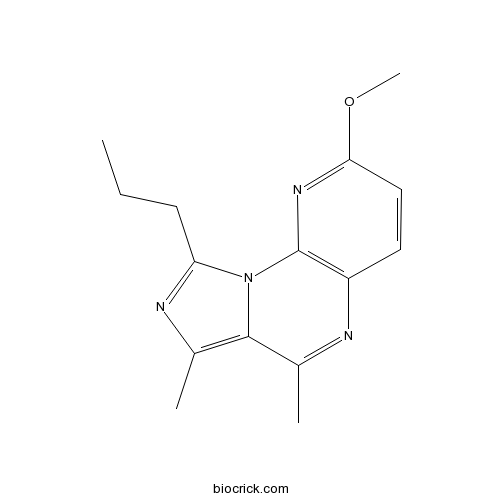
| SMILES | CCCC1=NC(=C2N1C3=C(C=CC(=N3)OC)N=C2C)C | ||
| Standard InChIKey | YNADXFWEXJTQSZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H18N4O/c1-5-6-12-17-10(3)14-9(2)16-11-7-8-13(20-4)18-15(11)19(12)14/h7-8H,5-6H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective PDE10A inhibitor (IC50 values are 7.28, 239, 779, 919, 3100 and 3700 nM at PDE10A, 2A, 11A, 5A, 7B and 3A respectively and >5000 nM at PDE1B, 4A, 6, 8A and 9A). Reverses MK 801-induced hyperactivity in vivo. |

TC-E 5005 Dilution Calculator

TC-E 5005 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6992 mL | 18.4959 mL | 36.9918 mL | 73.9836 mL | 92.4796 mL |
| 5 mM | 0.7398 mL | 3.6992 mL | 7.3984 mL | 14.7967 mL | 18.4959 mL |
| 10 mM | 0.3699 mL | 1.8496 mL | 3.6992 mL | 7.3984 mL | 9.248 mL |
| 50 mM | 0.074 mL | 0.3699 mL | 0.7398 mL | 1.4797 mL | 1.8496 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.3699 mL | 0.7398 mL | 0.9248 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7,4-Trihydroxy-3,6-dimethoxy-3-prenylflavone
Catalog No.:BCN1297
CAS No.:959421-20-6
- 4-Hydroxyalternariol 9-methyl ether
Catalog No.:BCN7389
CAS No.:959417-17-5
- PF 750
Catalog No.:BCC7641
CAS No.:959151-50-9
- A922500
Catalog No.:BCC2333
CAS No.:959122-11-3
- 2-Benzoylacetanilide
Catalog No.:BCC8560
CAS No.:959-66-0
- Sotalol hydrochloride
Catalog No.:BCC5165
CAS No.:959-24-0
- 12-Hydroxy-8(17),13-labdadien-16,15-olide
Catalog No.:BCN1298
CAS No.:958885-86-4
- GSK1059615
Catalog No.:BCC4984
CAS No.:958852-01-2
- SID 26681509
Catalog No.:BCC2362
CAS No.:958772-66-2
- (E)-1-(4-Hydroxyphenyl)dec-1-en-3-one
Catalog No.:BCN4031
CAS No.:958631-84-0
- Momordin IIa
Catalog No.:BCN3474
CAS No.:95851-50-6
- Momordin II
Catalog No.:BCN3473
CAS No.:95851-41-5
- Guggulsterone Z
Catalog No.:BCN3793
CAS No.:95975-55-6
- CH5138303
Catalog No.:BCC5364
CAS No.:959763-06-5
- Guajadial
Catalog No.:BCN4509
CAS No.:959860-49-2
- Aminothiazole
Catalog No.:BCC4623
CAS No.:96-50-4
- Mepivacaine
Catalog No.:BCC9020
CAS No.:96-88-8
- 2-hexyl-4-Pentynoic Acid
Catalog No.:BCC6480
CAS No.:96017-59-3
- SD 1008
Catalog No.:BCC2442
CAS No.:960201-81-4
- Vortioxetine (Lu AA21004) HBr
Catalog No.:BCC1213
CAS No.:960203-27-4
- Meropenem
Catalog No.:BCC2489
CAS No.:96036-03-2
- ONX-0914 (PR-957)
Catalog No.:BCC2095
CAS No.:960374-59-8
- Jatrorrhizine Hydrochloride
Catalog No.:BCC8193
CAS No.:960383-96-4
- ent-17-Hydroxykauran-3-one
Catalog No.:BCN4510
CAS No.:960589-81-5
Inhibition of Adrenergic and Non-Adrenergic Smooth Muscle Contraction in the Human Prostate by the Phosphodiesterase 10-Selective Inhibitor TC-E 5005.[Pubmed:27418235]
Prostate. 2016 Nov;76(15):1364-74.
BACKGROUND: The phosphodiesterase (PDE) 5 inhibitor tadalafil is available for treatment of male lower urinary tract symptoms (LUTS), while the role of other PDE isoforms for prostate smooth muscle tone is still unknown. Here, we examined effects of the PDE10-selective inhibitor TC-E 5005 on smooth muscle contraction in human prostate tissue. METHODS: Prostate samples were obtained from patients undergoing radical prostatectomy. Expression of PDE10 was addressed by RT-PCR, Western blot, and fluorescence staining with different markers. Effects of TC-E 5005 and tadalafil on contraction, and relaxation of prostate strips were studied via organ bath. RESULTS: PDE10A was detectable by RT-PCR, Western blot, and fluorescence staining in prostate tissues. Colocalization with markers suggested expression of PDE10A in smooth muscle cells and catecholaminergic nerves. Norepinephrine, the alpha1 -adrenergic agonist phenylephrine, the thromboxane A2 analogue U46619, and endothelins 1-3 induced concentration-dependent contractions of prostate strips, while electric field stimulation (EFS) induced frequence-dependent contractions. Application of TC-E 5005 (500 nM) caused significant inhibition of norepinephrine-, phenylephrine-, and endothelin-3-induced contractions. Inhibition of EFS-induced contractions by TC-E 5005 ranged around 50%, resembling inhibition of EFS-induced contractions by tadalafil (10 muM). The prostacyclin analog treprostinil and the nitric oxide donor DEA NONOate induced relaxations of precontracted prostate strips, which were significantly amplified by TCE 5005. CONCLUSIONS: The PDE10-selective inhibitor TC-E 5005 inhibits adrenergic and neurogenic smooth muscle contractions in the human prostate. TC-E 5005 inhibits neurogenic contractions with similar efficacy than tadalafil, so that urodynamic effects in vivo appear possible. Prostate 76:1364-1374, 2016. (c) 2016 Wiley Periodicals, Inc.
Discovery of imidazo[1,5-a]pyrido[3,2-e]pyrazines as a new class of phosphodiesterase 10A inhibitiors.[Pubmed:20450197]
J Med Chem. 2010 Jun 10;53(11):4399-411.
Novel imidazo[1,5-a]pyrido[3,2-e]pyrazines have been synthesized and characterized as both potent and selective phosphodiesterase 10A (PDE10A) inhibitors. For in vitro characterization, inhibition of PDE10A mediated cAMP hydrolysis was used and a QSAR model was established to analyze substitution effects. The outcome of this analysis was complemented by the crystal structure of PDE10A in complex with compound 49. Qualitatively new interactions between inhibitor and binding site were found, contrasting with previously published crystal structures of papaverine-like inhibitors. In accordance with the known antipsychotic potential of PDE10A inhibitors, MK-801 induced stereotypy and hyperactivity in rats were reversed by selected compounds. Thus, a promising compound class has been identified for the treatment of schizophrenia that could circumvent side effects connected with current therapies.


