4-HydroxyderricinCAS# 55912-03-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55912-03-3 | SDF | Download SDF |
| PubChem ID | 6438503.0 | Appearance | Powder |
| Formula | C21H22O4 | M.Wt | 338.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
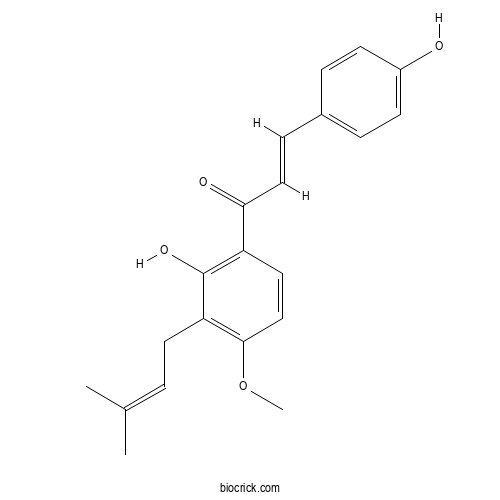
| Chemical Name | (E)-1-[2-hydroxy-4-methoxy-3-(3-methylbut-2-enyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one | ||
| SMILES | CC(=CCC1=C(C=CC(=C1O)C(=O)C=CC2=CC=C(C=C2)O)OC)C | ||
| Standard InChIKey | XDKYBPGIBVMHHB-KPKJPENVSA-N | ||
| Standard InChI | InChI=1S/C21H22O4/c1-14(2)4-10-18-20(25-3)13-11-17(21(18)24)19(23)12-7-15-5-8-16(22)9-6-15/h4-9,11-13,22,24H,10H2,1-3H3/b12-7+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

4-Hydroxyderricin Dilution Calculator

4-Hydroxyderricin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9551 mL | 14.7754 mL | 29.5508 mL | 59.1017 mL | 73.8771 mL |
| 5 mM | 0.591 mL | 2.9551 mL | 5.9102 mL | 11.8203 mL | 14.7754 mL |
| 10 mM | 0.2955 mL | 1.4775 mL | 2.9551 mL | 5.9102 mL | 7.3877 mL |
| 50 mM | 0.0591 mL | 0.2955 mL | 0.591 mL | 1.182 mL | 1.4775 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2955 mL | 0.591 mL | 0.7388 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- α-Cannabispiranol
Catalog No.:BCX1628
CAS No.:69636-83-5
- N,N'-dimethyldaurisoline iodide
Catalog No.:BCX1627
CAS No.:1422186-34-2
- 10,11-Dimethoxy-17-epi-alpha-yohimbine
Catalog No.:BCX1626
CAS No.:84667-06-1
- 3,4-Dimethoxycinnamic Acid
Catalog No.:BCX1625
CAS No.:14737-89-4
- Luteolin 7-galacturonide
Catalog No.:BCX1624
CAS No.:56324-53-9
- Luteolin 3'-galacturonide
Catalog No.:BCX1623
CAS No.:56317-12-5
- Prostratin
Catalog No.:BCX1622
CAS No.:60857-08-1
- Caffeoylmalic Acid
Catalog No.:BCX1621
CAS No.:149197-97-7
- Bebeerine
Catalog No.:BCX1620
CAS No.:477-60-1
- 3-O-acetyloleanolicacetic anhydride
Catalog No.:BCX1619
CAS No.:4339-73-5
- 1,7-dihydroxy-3,4-dimethoxylxanthone-7-O-Veratriloside
Catalog No.:BCX1618
CAS No.:76907-78-3
- 3'-Methoxy-5'-hydroxy isoflavone-7-O-beta-D-glucoside
Catalog No.:BCX1617
CAS No.:241129-90-8
- (2β,3β,4α,16α)-2,3,16,23-Tetrahydroxyolean-13(18)-en-28-oic acid
Catalog No.:BCX1630
CAS No.:2226630-77-7
- 12β-Acetoxyganoderic Acid θ
Catalog No.:BCX1631
CAS No.:2374206-90-1
- 2-[3-(3,7-Dimethyl-2,6-octadien-1-yl)-2,4-dihydroxyphenyl]-5,7-dihydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX1632
CAS No.:2226467-12-3
- (7aR,8R,13aR,13bS,13cR)-Dodecahydro-8-hydroxy-1H,5H,10H-dipyrido[2,1-f:3',2',1'-ij][1,6]naphthyridin...
Catalog No.:BCX1633
CAS No.:2306139-04-6
- Epiberberine chloride
Catalog No.:BCX1634
CAS No.:889665-86-5
- BioCrick001
Catalog No.:BCX1635
CAS No.:2600799-45-7
- 10-O-trans-p-methoxycinnamoyl-catalpol
Catalog No.:BCX1636
CAS No.:201605-27-8
- 10-O-[(E)-3,4-Dimethoxycinnamoyl]-catalpol
Catalog No.:BCX1637
CAS No.:834155-36-1
- 1,3-Dilinoleoyl-2-oleoylglycerol
Catalog No.:BCX1638
CAS No.:2190-22-9
- (2,4-dichloro-3,5-dimethoxyphenyl)methyl 6-O-β-D-glucopyranosyl-β-D-glucopyranoside
Catalog No.:BCX1639
CAS No.:2739844-79-0
- 4-chloro-3-methoxy-5-methylphenyl 6-O-(6-deoxy-β-L-mannopyranosyl)-β-D-glucopyranoside
Catalog No.:BCX1640
CAS No.:2839363-15-2
- (2,4,6-trichloro-3-hydroxy-5-methoxyphenyl)methyl β-D-glucopyranoside
Catalog No.:BCX1641
CAS No.:2839363-14-1
Randomised, double-blind, parallel group comparison of Ashitaba (Angelica Keiskei) chalcone effects on visceral fat areas and waist circumference of overweight persons.[Pubmed:38557440]
Int J Food Sci Nutr. 2024 Apr 1:1-10.
This randomised, placebo-controlled, double-blind, parallel-group study aimed to determine whether encapsulated Ashitaba chalcone (16 mg comprising 10.1 mg 4-Hydroxyderricin and 5.9 mg xanthoangelol) could reduce obesity in 17 men and 25 women with a body mass index (BMI) of 25 to < 30. Participants ingested capsules containing either the chalcone or a placebo daily for 12 weeks. The primary endpoint was changes in visceral fat areas determined by computed tomography (CT) at baseline, and at 8 and 12 weeks later. The primary endpoint, abdominal visceral fat area, was significantly reduced in the chalcone, compared with a placebo group 12 weeks after screening (p < 0.05). The secondary endpoint, waist circumference, was significantly decreased in the chalcone, compared with the placebo group at weeks 8 and 12 (p < 0.05 at week 8; p < 0.01 at week 12). Therefore, Ashitaba chalcone has anti-obesity benefits for overweight men and women.
The pharmacology activities of Angelica keiskei Koidzumi and its efficacy and safety in humans.[Pubmed:38357325]
Heliyon. 2024 Jan 13;10(2):e24119.
Chronic exposure to elevated levels of pro-oxidant factors may cause structural failings at the mitochondrial DNA level and alteration of antioxidant enzymes (glutathione peroxidase, catalase, and superoxide dismutase). Oxidative stress is an imbalance between the capacity of endogenous non-enzymatic antioxidants (glutathione, alpha-lipoic acid, uric acid, ferritin, metallothionein, melatonin, and bilirubin) and the occurrence of pro-oxidant factors which may lead to the pathogenesis of various diseases that affects the kidneys, pancreas, central nervous system, and cardiovascular system. Therefore, the utilization of medicinal plants with antioxidant activity, e.g., Angelica keiskei Koidzumi which contains chalcones, is interesting to be explored. Chalcones exhibit direct and indirect antioxidant activity and prevent oxidative stress by decreasing ROS, RNS, and superoxide production. In this review, we discuss the pharmacology activities of A. keiskei Koidzumi and its efficacy in humans. The articles were explored on PubMed and Google Scholar databases and based on the titles and abstracts related to the topic of interest, and 55 articles were selected. Two main chalcones of this plant, 4-Hydroxyderricin and xanthoangelol, have been reported for their various pharmacology activities. The efficacy of A. keiskei was confirmed in anti-obesity, hepatoprotective, anti-diabetes mellitus, and increasing plasma antioxidants in patients with metabolic syndrome. A keiskei is safe as proven by only mild or no adverse events reported, thus it is prospective to be further developed as an antioxidant nutraceutical.
Two Prenylated Chalcones, 4-Hydroxyderricin, and Xanthoangelol Prevent Postprandial Hyperglycemia by Promoting GLUT4 Translocation via the LKB1/AMPK Signaling Pathway in Skeletal Muscle Cells.[Pubmed:38267744]
Mol Nutr Food Res. 2024 Mar;68(5):e2300538.
SCOPE: Stimulation of glucose uptake in the skeletal muscle is crucial for the prevention of postprandial hyperglycemia. Insulin and certain polyphenols enhance glucose uptake through the translocation of glucose transporter 4 (GLUT4) in the skeletal muscle. The previous study reports that prenylated chalcones, 4-Hydroxyderricin (4-HD), and xanthoangelol (XAG) promote glucose uptake and GLUT4 translocation in L6 myotubes, but their underlying molecular mechanism remains unclear. This study investigates the mechanism in L6 myotubes and confirms antihyperglycemia by 4-HD and XAG. METHODS AND RESULTS: In L6 myotubes, 4-HD and XAG promote glucose uptake and GLUT4 translocation through the activation of adenosine monophosphate-activated protein kinase (AMPK) and liver kinase B1 (LKB1) signaling pathway without activating phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and Janus kinases (JAKs)/signal transducers and activators of transcriptions (STATs) pathways. Moreover, Compound C, an AMPK-specific inhibitor, as well as siRNA targeting AMPK and LKB1 completely canceled 4-HD and XAG-increased glucose uptake. Consistently, oral administration of 4-HD and XAG to male ICR mice suppresses acute hyperglycemia in an oral glucose tolerance test. CONCLUSION: In conclusion, LKB1/AMPK pathway and subsequent GLUT4 translocation in skeletal muscle cells are involved in Ashitaba chalcone-suppressed acute hyperglycemia.
Effects of Microbial Transformation on the Biological Activities of Prenylated Chalcones from Angelica keiskei.[Pubmed:35206019]
Foods. 2022 Feb 14;11(4):543.
Microbial transformation is an alternative method for structural modification. The current study aimed at application of microbial transformation for discovering new derivatives and investigating the structure-activity relationship of isobavachalcone (1), 4-Hydroxyderricin (2), and xanthoangelol (3) isolated from the herb Angelica keiskei. In the initial screening process, 1-3 were incubated with microbes using a two-stage fermentation method and analyzed through TLC monitoring. The screening results showed that Rhizopus oryzae and Mucor hiemalis were able to transform 1 and 2, respectively. Additionally, M. hiemalis and Mortierella ramanniana var. angulispora were able to transform 3. Following scale-up fermentation, four new (4, 5, 7, and 10) and five known (6, 8, 9, 11, and 12) metabolites were produced. Cytotoxicity of all the compounds (1-12) was investigated using three human cancer cell lines including A375P, HT-29, and MCF-7 by MTT method. Meanwhile, the tyrosinase inhibitory activity of 1-12 was evaluated using l-tyrosine as a substrate. Overall, 1 and 3 displayed the highest cytotoxicity, and 5 and 7 exhibited the most potent tyrosinase inhibitory activity with relatively low cytotoxicity. This allowed us to postulate that the introduction of 4'-O-glucopyranosyl group led to the reduction in cytotoxicity and improvement in tyrosinase inhibitory activity.
Chalcones from Angelica keiskei (ashitaba) inhibit key Zika virus replication proteins.[Pubmed:35124513]
Bioorg Chem. 2022 Mar;120:105649.
Zika virus (ZIKV) is a dangerous human pathogen and no antiviral drugs have been approved to date. The chalcones are a group of small molecules that are found in a number of different plants, including Angelica keiskei Koidzumi, also known as ashitaba. To examine chalcone anti-ZIKV activity, three chalcones, 4-Hydroxyderricin (4HD), xanthoangelol (XA), and xanthoangelol-E (XA-E), were purified from a methanol-ethyl acetate extract from A. keiskei. Molecular and ensemble docking predicted that these chalcones would establish multiple interactions with residues in the catalytic and allosteric sites of ZIKV NS2B-NS3 protease, and in the allosteric site of the NS5 RNA-dependent RNA-polymerase (RdRp). Machine learning models also predicted 4HD, XA and XA-E as potential anti-ZIKV inhibitors. Enzymatic and kinetic assays confirmed chalcone inhibition of the ZIKV NS2B-NS3 protease allosteric site with IC(50)s from 18 to 50 microM. Activity assays also revealed that XA, but not 4HD or XA-E, inhibited the allosteric site of the RdRp, with an IC(50) of 6.9 microM. Finally, we tested these chalcones for their anti-viral activity in vitro with Vero cells. 4HD and XA-E displayed anti-ZIKV activity with EC(50) values of 6.6 and 22.0 microM, respectively, while XA displayed relatively weak anti-ZIKV activity with whole cells. With their simple structures and relative ease of modification, the chalcones represent attractive candidates for hit-to-lead optimization in the search of new anti-ZIKV therapeutics.
4-Hydroxyderricin Promotes Apoptosis and Cell Cycle Arrest through Regulating PI3K/AKT/mTOR Pathway in Hepatocellular Cells.[Pubmed:34574146]
Foods. 2021 Aug 29;10(9):2036.
4-Hydroxyderricin (4-HD), as a natural flavonoid compound derived from Angelica keiskei, has largely unknown inhibition and mechanisms on liver cancer. Herein, we investigated the inhibitory effects of 4-HD on hepatocellular carcinoma (HCC) cells and clarified the potential mechanisms by exploring apoptosis and cell cycle arrest mediated via the PI3K/AKT/mTOR signaling pathway. Our results show that 4-HD treatment dramatically decreased the survival rate and activities of HepG2 and Huh7 cells. The protein expressions of apoptosis-related genes significantly increased, while those related to the cell cycle were decreased by 4-HD. 4-HD also down-regulated PI3K, p-PI3K, p-AKT, and p-mTOR protein expression. Moreover, PI3K inhibitor (LY294002) enhanced the promoting effect of 4-HD on apoptosis and cell cycle arrest in HCC cells. Consequently, we demonstrate that 4-HD can suppress the proliferation of HCC cells by promoting the PI3K/AKT/mTOR signaling pathway mediated apoptosis and cell cycle arrest.
EPRS/GluRS promotes gastric cancer development via WNT/GSK-3beta/beta-catenin signaling pathway.[Pubmed:33740160]
Gastric Cancer. 2021 Sep;24(5):1021-1036.
BACKGROUND: Glutamyl-prolyl-tRNA synthetase (EPRS/GluRS) is primarily part of the multi-synthetase complex that may play a key role in cancer development. However, the biological function, molecular mechanism, and inhibitor of EPRS have not been investigated in gastric cancer (GC). METHODS: Immunohistochemistry was performed to detect the expression of EPRS in human gastric tumor tissues. Knocking down of EPRS, cell-derived xenograft mouse model, and patient-derived xenograft mouse model was used to identify the biological function of EPRS. Immunoprecipitation was applied to elucidate the interaction between EPRS and SCYL2. Computer docking model and multiple in vitro and in vivo experiments were conducted to discover EPRS inhibitors. RESULTS: Here, we report that EPRS is frequently overexpressed in GC tissues compared to that adjacent controls and its overexpression predicts poor prognosis in GC patients. Functionally, high expression of EPRS positively co-relates with GC development both in vitro and in vivo. Mechanistically, EPRS directly binds with SCYL2 to enhance the activation of WNT/GSK-3beta/beta-catenin signaling pathway and the accumulation of beta-catenin in the nuclear, leading to GC cell proliferation and tumor growth. Moreover, we identified that xanthoangelol (XA) and 4-Hydroxyderricin (4-HD) can directly bind to EPRS to block WNT/GSK-3beta/beta-catenin signaling pathway. More importantly, XA and 4-HD restrain gastric cancer patient-derived xenograft tumor growth and Helicobacter pylori combined with alcohol-induced atrophic gastritis and gastric tumorigenesis. CONCLUSION: These findings unveil a promising strategy for GC prevention and therapy by targeting EPRS-mediated WNT/GSK-3beta/beta-catenin cascades. Moreover, XA and 4-HD may be effective reagents used for GC prevention and therapy.
4-Hydroxyderricin and xanthoangelol isolated from Angelica keiskei prevent dexamethasone-induced muscle loss.[Pubmed:32510085]
Food Funct. 2020 Jun 24;11(6):5498-5512.
Since a decrease in muscle mass leads to an increased risk of mortality, the prevention of muscle wasting contributes to maintaining the quality of life. Recently, we reported that glabridin, a prenylated flavonoid in licorice, prevents dexamethasone-induced muscle loss. In this study, we focused on the other prenylated chalcones 4-Hydroxyderricin and xanthoangelol in Ashitaba (Angelica keiskei) and investigated their prevention effect on dexamethasone-induced muscle loss. It was found that 4-Hydroxyderricin and xanthoangelol significantly prevented dexamethasone-induced protein degradation in C2C12 myotubes by suppressing the expression of ubiquitin ligases, Cbl-b and MuRF-1. These prenylated chalcones acted as the antagonists of the glucocorticoid receptor and inhibited the binding of dexamethasone to this receptor and its subsequent nuclear translocation. In addition, the chalcones suppressed the phosphorylation of p38 and FoxO3a as the upstream factors for ubiquitin ligases. Dexamethasone-induced protein degradation and upregulation of Cbl-b were attenuated by the knockdown of the glucocorticoid receptor but not by the knockdown of p38. In male C57BL/6J mice, the Ashitaba extract, containing 4-Hydroxyderricin and xanthoangelol, suppressed dexamethasone-induced muscle mass wasting accompanied by a decrease in the expression of ubiquitin ligases by inhibiting the nuclear translocation of the glucocorticoid receptor and phosphorylation of FoxO3a. In conclusion, 4-Hydroxyderricin and xanthoangelol are effective compounds to inhibit steroid-induced muscle loss.
A Chalcone from Ashitaba (Angelica keiskei) Stimulates Myoblast Differentiation and Inhibits Dexamethasone-Induced Muscle Atrophy.[Pubmed:31658768]
Nutrients. 2019 Oct 10;11(10):2419.
Ashitaba, Angelica keiskei Koidzumi (AK), as a traditional medicine in Korea, Japan, and China, has been known as an elixir of life having therapeutic potential. However, there is no scientific evidence to support that Ashitaba can enhance or maintain muscle strength. To find a new therapeutic agent from the medicinal plant, we evaluated the anti-myopathy effect of chalcones from ethanol extract of AK (EAK) in cellular and animal models of muscle atrophy. To examine anti-myopathy activity, EAK was treated into dexamethasone injected rats and muscle thickness and histopathological images were analyzed. Oral administration of EAK (250 or 500 mg/kg) alleviated muscle atrophic damages and down-regulated the mRNA levels of muscle-specific ubiquitin-E3 ligases. Among ten compounds isolated from EAK, 4-Hydroxyderricin was the most effective principle in stimulating myogenesis of C2C12 myoblasts via activation of p38 mitogen-activated protein kinase (MAPK). In three cellular muscle atrophy models with C2C12 myoblasts damaged by dexamethasone or cancer cell-conditioned medium, 4-Hydroxyderricin protected the myosin heavy chain (MHC) degradation through suppressing expressions of MAFbx, MuRF-1 and myostatin. These results suggest that the ethanol extract and its active principle, 4-Hydroxyderricin from AK, can overcome the muscle atrophy through double mechanisms of decreasing muscle protein degradation and activating myoblast differentiation.
Characterizing Tyrosinase Modulators from the Roots of Angelica keiskei Using Tyrosinase Inhibition Assay and UPLC-MS/MS as the Combinatorial Novel Approach.[Pubmed:31510069]
Molecules. 2019 Sep 10;24(18):3297.
In this study, an in vitro tyrosinase inhibition assay in combination with ultra performance liquid chromatography-orbitrap mass spectrometry (UPLC-orbitrap-MS) was developed for the rapid screening and identification of tyrosinase modulators from roots of Angelica keiskei. Of the 15 candidates considered, nine chalcones, xanthoangelols (1), B (2), D (3), E (4), G (5), H (6), 4-Hydroxyderricin (7), xanthokeismin B (8) and (2E)-1-[4-hydroxy-2-(2-hydroxy-2-propanyl)-2,3-dihydro-1-benzofuran-7-yl]-3-(4-hydroxyphenyl)-2-propen-1-one (9), five coumarins, umbelliferone (10), selinidin (11), isopimpinellin (12), phellopterin (13) and xanthyletin (14), and one other compound, ashitabaol A (15), were distinguished between the test samples and the controls with statistical significance, and the structure of each compound was determined by comparing with in-house standards and the literature. Among these, six compounds, xanthoangelol (1), xanthoangelol D (3), xanthoangelol H (6), 4-Hydroxyderricin (7), laserpitin (16) and isolaserpitin (17), were isolated from roots of A. keiskei. Of the compounds isolated, compounds 1, 7 and 16 were subjected to tyrosinase inhibitory assay, and the IC(50) values were 15.87 +/- 1.21, 60.14 +/- 2.29 and >100 muM, respectively. The present study indicated that the combination of in vitro tyrosinase inhibition assay coupled with UPLC-MS/MS could be widely applied to the rapid screening of active substances from various natural resources.
Analysis of plasma metabolic profiling and evaluation of the effect of the intake of Angelica keiskei using metabolomics and lipidomics.[Pubmed:31283957]
J Ethnopharmacol. 2019 Oct 28;243:112058.
ETHNOPHARMACOLOGICAL RELEVANCE: Angelica keiskei contains many bioactive components with anti-oxidative and anti-inflammatory effects. It is also effective for the treatment of diabetes mellitus, hypertension, and arteriosclerosis, but the relationships between these effects and the active components in the herb have not been studied. AIM OF THE STUDY: We aimed to confirm the effects of Angelica keiskei on humans. MATERIALS AND METHODS: A metabolomics and lipidomics study was performed using human plasma samples from 20 subjects after the intake of Angelica keiskei, and the components of Angelica keiskei in the plasma were profiled. UPLC-Orbitrap-MS was used to analyze the plasma and plant extracts, and multivariate analysis and correlation studies between the exogenous components from plant and endogenous metabolite in plasma were performed. RESULTS: The levels of the 14 metabolites including kynurenic acid, prostaglandin E1, chenodeoxycholic acid, lysoPC (18:1), lysoPC (18:2), lysoPC (20:3), lysoPC (20:4), lysoPC (22:6), PC (34:1), PC (34:2), PC (38:3), PC (38:4), PC (38:6) and PC (40:7) in the plasma were changed. By monitoring the components originating from Angelica keiskei in plasma, five components including 5-methoxypsoralen, 8-methoxypsoralen, 4-Hydroxyderricin, xanthoangelol B and xanthoangelol F were detected and they reduced the levels of bile acids and fatty acids. CONCLUSIONS: The levels of the metabolites, including bile acids, amino acids, glycerophospholipids and fatty acids, in the plasma were changed, and 14 significantly changed metabolites were closely related to the preventive effect against liver diseases, type 2 diabetes, anemia, obesity, atherosclerosis, depression and anti-inflammatory effects. The five components of Angelica keiskei were related the modulatory activity of reducing the levels of bile acids and fatty acids.
4-Hydroxyderricin inhibits osteoclast formation and accelerates osteoblast differentiation.[Pubmed:30474804]
Cytotechnology. 2019 Feb;71(1):15-22.
4-Hydroxyderricin (4-HD) is a major polyphenol of Angelica keiskei (Japanese name Ashitaba), exhibiting anti-allergic, anti-diabetic, anti-oxidant, and antitumor effects. The present study was designed to evaluate the effects of 4-HD on bone formation and maintenance by using cultured osteoclasts and osteoblasts. 4-HD did not affect cell proliferation of stromal ST2 cells and preosteoblast MC3T3-E1 cells at concentrations of 1-10 muM. This compound inhibited the formation of multinucleated osteoclasts from mouse splenic cells, and we identified a molecular pathway of osteoclast differentiation mediated by 4-HD, which led to inhibition of the expression of receptor activator of nuclear factor-kappaB ligand and macrophage-colony stimulating factor in ST2 cells. By contrast, 4-HD enhanced indices of osteoblast differentiation, such as alkaline phosphatase activity and calcium deposition by osteoblastic MC3T3-E1 cells, at concentrations of 1-10 muM. Furthermore, we found that 4-HD at 1 muM attenuated H(2)O(2) levels in MC3T3-E1 cells. Our findings indicate that 4-HD may have critical effects on bone formation and maintenance.
The Ashitaba (Angelica keiskei) Chalcones 4-hydroxyderricin and Xanthoangelol Suppress Melanomagenesis By Targeting BRAF and PI3K.[Pubmed:29980517]
Cancer Prev Res (Phila). 2018 Oct;11(10):607-620.
Malignant melanoma is an aggressive tumor of the skin and still lacks effective preventive and therapeutic treatments. In melanoma, both the BRAF/MEK/ERK and PI3-K/AKT signaling pathways are constitutively activated through multiple mechanisms, which result in cell-cycle progression and prevention of apoptosis. Therefore, the development of novel strategies for targeting BRAF and PI3K are of utmost importance. In this study, we found that Ashitaba (Angelica keiskei) chalcones, 4-Hydroxyderricin (4HD) and xanthoangelol (XAG), suppressed melanoma development by directly targeting both BRAFV600E and PI3K, which blocked the activation of downstream signaling. This led to the induction of G(1) phase cell-cycle arrest and apoptosis in melanoma cells. Importantly, 4HD or XAG dramatically attenuated tumor incidence and volume in the BRAF-activated Pten-deficient melanoma mouse model. Our findings suggest that 4HD and XAG are promising chemopreventive or potential therapeutic agents against melanomagenesis that act by targeting both BRAF and PI3K, providing hope for rapid clinical translation. Cancer Prev Res; 11(10); 607-20. (c)2018 AACR.
Integration of Biochemometrics and Molecular Networking to Identify Antimicrobials in Angelica keiskei.[Pubmed:29571174]
Planta Med. 2018 Jul;84(9-10):721-728.
Botanical medicines have been utilized for centuries, but it remains challenging to identify bioactive constituents from complex botanical extracts. Bioassay-guided fractionation is often biased toward abundant or easily isolatable compounds. To comprehensively evaluate active botanical mixtures, methods that allow for the prioritization of active compounds are needed. To this end, a method integrating bioassay-guided fractionation, biochemometric selectivity ratio analysis, and molecular networking was devised and applied to Angelica keiskei to comprehensively evaluate its antimicrobial activity against Staphylococcus aureus. This approach enabled the identification of putative active constituents early in the fractionation process and provided structural information for these compounds. A subset of chalcone analogs were prioritized for isolation, yielding 4-Hydroxyderricin (1, minimal inhibitory concentration [MIC]


