BebeerineCAS# 477-60-1 |
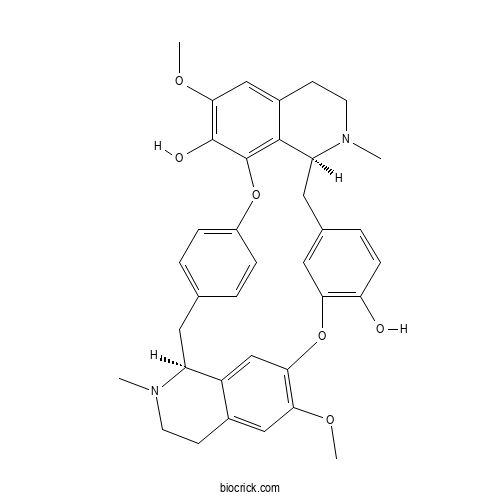
- (-)-Curine
Catalog No.:BCN2673
CAS No.:436-05-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 477-60-1 | SDF | Download SDF |
| PubChem ID | 12300019.0 | Appearance | Powder |
| Formula | C36H38N2O6 | M.Wt | 594.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Curine; Pelosine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,16S)-10,25-dimethoxy-15,30-dimethyl-7,23-dioxa-15,30-diazaheptacyclo[22.6.2.23,6.18,12.118,22.027,31.016,34]hexatriaconta-3(36),4,6(35),8(34),9,11,18(33),19,21,24,26,31-dodecaene-9,21-diol | ||
| SMILES | CN1CCC2=CC(=C3C=C2C1CC4=CC=C(C=C4)OC5=C6C(CC7=CC(=C(C=C7)O)O3)N(CCC6=CC(=C5O)OC)C)OC | ||
| Standard InChIKey | NGZXDRGWBULKFA-NSOVKSMOSA-N | ||
| Standard InChI | InChI=1S/C36H38N2O6/c1-37-13-11-23-18-31(41-3)32-20-26(23)27(37)15-21-5-8-25(9-6-21)43-36-34-24(19-33(42-4)35(36)40)12-14-38(2)28(34)16-22-7-10-29(39)30(17-22)44-32/h5-10,17-20,27-28,39-40H,11-16H2,1-4H3/t27-,28-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bebeerine Dilution Calculator

Bebeerine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6815 mL | 8.4075 mL | 16.8149 mL | 33.6298 mL | 42.0373 mL |
| 5 mM | 0.3363 mL | 1.6815 mL | 3.363 mL | 6.726 mL | 8.4075 mL |
| 10 mM | 0.1681 mL | 0.8407 mL | 1.6815 mL | 3.363 mL | 4.2037 mL |
| 50 mM | 0.0336 mL | 0.1681 mL | 0.3363 mL | 0.6726 mL | 0.8407 mL |
| 100 mM | 0.0168 mL | 0.0841 mL | 0.1681 mL | 0.3363 mL | 0.4204 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-O-acetyloleanolicacetic anhydride
Catalog No.:BCX1619
CAS No.:4339-73-5
- 1,7-dihydroxy-3,4-dimethoxylxanthone-7-O-Veratriloside
Catalog No.:BCX1618
CAS No.:76907-78-3
- 3'-Methoxy-5'-hydroxy isoflavone-7-O-beta-D-glucoside
Catalog No.:BCX1617
CAS No.:241129-90-8
- Eupalinilide A
Catalog No.:BCX1616
CAS No.:757202-06-5
- Lambertic Acid
Catalog No.:BCX1615
CAS No.:55051-96-2
- Ethyl-beta-D- glucoside
Catalog No.:BCX1614
CAS No.:19467-01-7
- Mesatlantin C
Catalog No.:BCX1613
CAS No.:137624-14-7
- Rathbunioside R1
Catalog No.:BCX1612
CAS No.:350689-78-0
- Ophiopogonin A
Catalog No.:BCX1611
CAS No.:11054-24-3
- Spinorhamnoside
Catalog No.:BCX1610
CAS No.:864271-19-2
- 4-Methoxyphenyl-beta-galactoside
Catalog No.:BCX1609
CAS No.:3150-20-7
- Nudol
Catalog No.:BCX1608
CAS No.:86630-46-8
- Caffeoylmalic Acid
Catalog No.:BCX1621
CAS No.:149197-97-7
- Prostratin
Catalog No.:BCX1622
CAS No.:60857-08-1
- Luteolin 3'-galacturonide
Catalog No.:BCX1623
CAS No.:56317-12-5
- Luteolin 7-galacturonide
Catalog No.:BCX1624
CAS No.:56324-53-9
- 3,4-Dimethoxycinnamic Acid
Catalog No.:BCX1625
CAS No.:14737-89-4
- 10,11-Dimethoxy-17-epi-alpha-yohimbine
Catalog No.:BCX1626
CAS No.:84667-06-1
- N,N'-dimethyldaurisoline iodide
Catalog No.:BCX1627
CAS No.:1422186-34-2
- α-Cannabispiranol
Catalog No.:BCX1628
CAS No.:69636-83-5
- 4-Hydroxyderricin
Catalog No.:BCX1629
CAS No.:55912-03-3
- (2β,3β,4α,16α)-2,3,16,23-Tetrahydroxyolean-13(18)-en-28-oic acid
Catalog No.:BCX1630
CAS No.:2226630-77-7
- 12β-Acetoxyganoderic Acid θ
Catalog No.:BCX1631
CAS No.:2374206-90-1
- 2-[3-(3,7-Dimethyl-2,6-octadien-1-yl)-2,4-dihydroxyphenyl]-5,7-dihydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX1632
CAS No.:2226467-12-3
Screening an In-House Isoquinoline Alkaloids Library for New Blockers of Voltage-Gated Na(+) Channels Using Voltage Sensor Fluorescent Probes: Hits and Biases.[Pubmed:35807390]
Molecules. 2022 Jun 28;27(13):4133.
Voltage-gated Na(+) (Na(V)) channels are significant therapeutic targets for the treatment of cardiac and neurological disorders, thus promoting the search for novel Na(V) channel ligands. With the objective of discovering new blockers of Na(V) channel ligands, we screened an In-House vegetal alkaloid library using fluorescence cell-based assays. We screened 62 isoquinoline alkaloids (IA) for their ability to decrease the FRET signal of voltage sensor probes (VSP), which were induced by the activation of Na(V) channels with batrachotoxin (BTX) in GH3b6 cells. This led to the selection of five IA: liriodenine, oxostephanine, thalmiculine, protopine, and Bebeerine, inhibiting the BTX-induced VSP signal with micromolar IC(50). These five alkaloids were then assayed using the Na(+) fluorescent probe ANG-2 and the patch-clamp technique. Only oxostephanine and liriodenine were able to inhibit the BTX-induced ANG-2 signal in HEK293-hNa(V)1.3 cells. Indeed, liriodenine and oxostephanine decreased the effects of BTX on Na(+) currents elicited by the hNa(V)1.3 channel, suggesting that conformation change induced by BTX binding could induce a bias in fluorescent assays. However, among the five IA selected in the VSP assay, only Bebeerine exhibited strong inhibitory effects against Na(+) currents elicited by the hNav1.2 and hNav1.6 channels, with IC(50) values below 10 microM. So far, Bebeerine is the first BBIQ to have been reported to block Na(V) channels, with promising therapeutical applications.
Comparative phytochemical investigation of the sources of ayurvedic drug patha: a chromatographic fingerprinting analysis.[Pubmed:20582188]
Indian J Pharm Sci. 2010 Jan;72(1):39-45.
Standardization of herbal drugs based on their chemical and biological activity profile is an important prerequisite for acquiring the herbal market. The main problem encountered in standardization of Ayurvedic drugs is proper identification of the source plant. The present study was aimed to establish identification characters, quality control parameters, chemical and biological parameters for roots of three plants Cissampelos pareira, Cyclea peltata and Stephania japonica (Fam. Menispermaceae) which are being used as source of Patha, in the market. All the three plant were subjected for evaluation of quality control parameters as per WHO guidelines and root extracts and total alkaloidal fractions were subjected for HPTLC and HPLC fingerprinting analysis using a marker compound Bebeerine isolated from roots of Cissampelos pareira. The parameters studied clearly indicated the significant differences among the three plant materials. The roots of Cissampelos pareira can be distinguished from other two plants by presence of high concentration of alkaloids especially the presence of high concentration of pharmacologically active alkaloid Bebeerine, which was found to be present in very low concentration in Stephania japonica and absent in roots of Cyclea peltata. The roots of Cyclea peltata were found to contain high concentration of saponins and comparatively in low concentration in Cissampelos pareira where as it was found to be absent in roots of Stephania japonica.
Natural probes for cholinergic sites: L-bebeerine actions on the neuromuscular transmission, the nicotinic receptor/ionic channel complex, and contraction of skeletal muscles.[Pubmed:10797870]
Acta Physiol Pharmacol Ther Latinoam. 1999;49(4):268-78.
The mechanisms underlying the muscle relaxant activity of 1-Bebeerine (BB), a tertiary alkaloid isolated from the roots of Chondrodendron platyphyllum, were examined in mammalian and amphibian skeletal muscles. Injections of BB (0.05-1 g/kg, i.p.) in rats caused a dose-related flaccid paralysis and respiratory arrest at high doses. In isolated rat diaphragm and toad sartorius muscles, BB depressed the indirectly elicited muscle twitches (IC50: 228 microM and 5.4 microM, respectively, at 22 degrees C) and blocked the nerve-elicited muscle action potential. The neuromuscular blockade was not reversed by neostigmine (10 microM). High concentrations of BB (170 and 340 microM) caused muscle contracture unrelated to the junctional blockade, and intensified by increasing the bath temperature. Analysis of the contraction properties showed that BB (40 and 80 microM) increased the twitch/tetanus ratio (46% and 125%) and prolonged the relaxation time; the falling phase of the directly elicited action potential in toad sartorius muscle fibers was slower probably by a decreased potassium conductance. BB (0.1-340 microM) reduced the binding of [125l]alpha--bungarotoxin to the junctional ACh receptor of the rat diaphragm (IC50: 47.7 microM, at 37 degrees C. At low concentrations BB (1.5-15 microM) induced either opening or blockade of the ACh receptor-ionic channel. The results showed that BB blocked noncompetitively the neuromuscular transmission through a mechanism that affects the ACh recognition site and the ionic channel properties. The alkaloid also produced muscle contracture and changed the contractile properties through its extra-junctional action at the calcium handling by the sarcoplasmic reticulum or the contractile machinery.


