NudolCAS# 86630-46-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 86630-46-8 | SDF | Download SDF |
| PubChem ID | 158975.0 | Appearance | Powder |
| Formula | C16H14O4 | M.Wt | 270.28 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 2,7-phenanthrenediol, 3,4-dimethoxy- | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
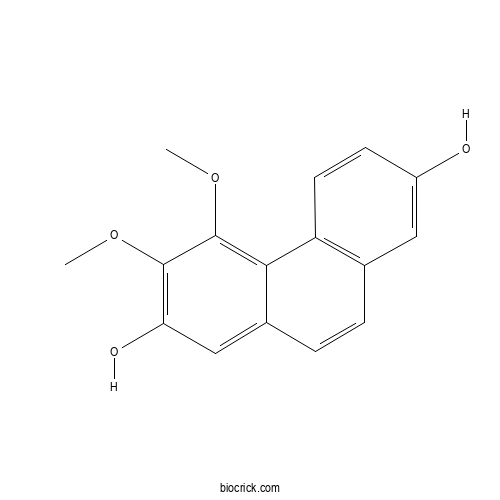
| Chemical Name | 3,4-dimethoxyphenanthrene-2,7-diol | ||
| SMILES | COC1=C(C=C2C=CC3=C(C2=C1OC)C=CC(=C3)O)O | ||
| Standard InChIKey | JZIYNZGPIKGKQC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H14O4/c1-19-15-13(18)8-10-4-3-9-7-11(17)5-6-12(9)14(10)16(15)20-2/h3-8,17-18H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Nudol Dilution Calculator

Nudol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6999 mL | 18.4993 mL | 36.9987 mL | 73.9973 mL | 92.4967 mL |
| 5 mM | 0.74 mL | 3.6999 mL | 7.3997 mL | 14.7995 mL | 18.4993 mL |
| 10 mM | 0.37 mL | 1.8499 mL | 3.6999 mL | 7.3997 mL | 9.2497 mL |
| 50 mM | 0.074 mL | 0.37 mL | 0.74 mL | 1.4799 mL | 1.8499 mL |
| 100 mM | 0.037 mL | 0.185 mL | 0.37 mL | 0.74 mL | 0.925 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-(4-methoxyphenyl)-3,4-dihydro-2H-chromen-7-ol
Catalog No.:BCX1607
CAS No.:10499-17-9
- 4-(3-oxobutyl)phenyl 6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-beta-D-glucopyranoside
Catalog No.:BCX1606
CAS No.:1609583-04-1
- L-Homoarginine hydrochloride
Catalog No.:BCX1605
CAS No.:1483-01-8
- L-Glutamic acid hydrochloride
Catalog No.:BCX1604
CAS No.:138-15-8
- Orientin-2''-O-p-trans-coumarate
Catalog No.:BCX1603
CAS No.:1229437-75-5
- L-Cysteine hydrochloride monohydrate
Catalog No.:BCX1602
CAS No.:7048-04-6
- Uvarimacrophin A
Catalog No.:BCX1601
CAS No.:167425-75-4
- Isobergaptol
Catalog No.:BCX1600
CAS No.:21339-45-7
- Schindilactone A
Catalog No.:BCX1599
CAS No.:943239-45-0
- Apigenin-7-O-glucuronide-6'-ethyl ester
Catalog No.:BCX1598
CAS No.:62268-42-2
- 14-Formyldihydrorutaecarpine
Catalog No.:BCX1597
CAS No.:68353-23-1
- Arisanlactone D
Catalog No.:BCX1596
CAS No.:1234186-01-6
- 4-Methoxyphenyl-beta-galactoside
Catalog No.:BCX1609
CAS No.:3150-20-7
- Spinorhamnoside
Catalog No.:BCX1610
CAS No.:864271-19-2
- Ophiopogonin A
Catalog No.:BCX1611
CAS No.:11054-24-3
- Rathbunioside R1
Catalog No.:BCX1612
CAS No.:350689-78-0
- Mesatlantin C
Catalog No.:BCX1613
CAS No.:137624-14-7
- Ethyl-beta-D- glucoside
Catalog No.:BCX1614
CAS No.:19467-01-7
- Lambertic Acid
Catalog No.:BCX1615
CAS No.:55051-96-2
- Eupalinilide A
Catalog No.:BCX1616
CAS No.:757202-06-5
- 3'-Methoxy-5'-hydroxy isoflavone-7-O-beta-D-glucoside
Catalog No.:BCX1617
CAS No.:241129-90-8
- 1,7-dihydroxy-3,4-dimethoxylxanthone-7-O-Veratriloside
Catalog No.:BCX1618
CAS No.:76907-78-3
- 3-O-acetyloleanolicacetic anhydride
Catalog No.:BCX1619
CAS No.:4339-73-5
- Bebeerine
Catalog No.:BCX1620
CAS No.:477-60-1
Nudol, a phenanthrene derivative from Dendrobium nobile, induces cell cycle arrest and apoptosis and inhibits migration in osteosarcoma cells.[Pubmed:31551653]
Drug Des Devel Ther. 2019 Jul 29;13:2591-2601.
Purpose: Osteosarcoma is the most common malignancy of the bone in children and adolescents. There is an urgent need for the development of novel drugs to treat it. Nudol(1), a phenanthrene compound from the traditional Chinese medicine, Dendrobium nobile, exhibited antiproliferative activity against osteosarcoma cells. Therefore, the aim of the present study was to investigate the role and underlying mechanism of Nudol(1) as potential chemotherapy for osteosarcoma. Methods: Cell viability was determined by MTT assay. Cell-cycle phase distribution was analyzed by flow cytometry and Western blot. DAPI staining was used for morphology observation. Apoptosis was analysis via flow cytometry. The expression levels of mRNA and protein related to capase-mediated apoptotic pathway were detected by real-time PCR and western blotting. Migration was determined by wound healing assays. Results: Nudol(1) significantly decreased cell viability in several cancer cell lines. Moreover, Nudol(1) caused cell cycle arrest at G2/M phase in U2OS cells, and it also induced cell apoptosis through the caspase-dependent pathway. In addition, treatment with Nudol(1) suppressed the migration of U2OS cells. Conclusion: The present study, for the first time, demonstrated effects of Nudol(1) on OS in vitro and the potential molecular mechanisms. Accordingly, Nudol(1) might have the potential for further development as a lead compound against bone tumor.
[Chemical constituents from tuber of Bletilla striata].[Pubmed:29071865]
Zhongguo Zhong Yao Za Zhi. 2017 Apr;42(8):1578-1584.
Eighteen compounds were isolated from the EtOAc soluble fraction of Bletilla striata by various chromatographic techniques, such as silica gel, ODS, Sephadex LH-20, RP-HPLC. Their structures were identified by spectroscopic methods and physicochemical properties, as 5-hydroxy-2-(p-hydroxybenzyl)- 3- methoxybibenzyl(1), shancigusins B(2), shanciguol(3),arundinan(4), 3',5-dihydroxy-2,4-di(p-hydroxybenzyl)-3-methoxybibenzyl(5), arundin(6), 3,3'-dihydroxy-2-(p-hydroxybenzyl)-5-methoxybibenzyl(7), 3, 3'-dihydroxy-2', 6'-bis(p-hydroxybenzyl)-5-methoxybibenzyl(8), 7-hydroxy-2,4-dimethoxyphenanthrene(9), bleformin B(10),Nudol(11), 3,7-dihydroxy-2, 4-dimethoxyphenanthrene(12), 2, 7-dihydroxy-4-methoxy-9,10-dihydrophenathrene(13), bleformin D(14), 4,4'-dimethoxy-9,10-dyhydro-[6,1'-biphenanthrene]-2,2',7,7'-tetraol(15),gymconopin C(16),(2,3-trans)-2-(4-hydroxy-3-methoxyphenyl)-3-hydroxymethyl-10-methoxy-2,3,4,5-tetrahydro-phenanthro[2,1-b]furan-7-ol(17),shanciol(18). Among them, compound 1 was a new compound, Compounds 2-6,9,15-18 were isolated from this genus for the first time.
Phenolic compounds from the stems of Flickingeria fimbriata.[Pubmed:28278646]
Nat Prod Res. 2017 Jul;31(13):1518-1522.
Chemical investigation of Flickingeria fimbriata (Bl.) Hawkes (Orchidaceae) resulted in the isolation and identification of one new dihydrophenanthrene, 1,2,5,6,7-pentamethoxy-9,10-dihydrophenanthrene (1), together with seven known dihydrophenanthrenes, erianthridin (2), coelonin (3), 4-methoxy-9,10-dihydrophenanthrene-1,2,7-triol (4), lusianthridin (5), ephemeranthol A (6), flavanthridin (7) and hircinol (8), four known phenanthrenes, epheranthol B (9), Nudol (10), denthyrsinin (11) and confusarin (12), and one known bibenzyl, batatasin III (13). The structure of the new compound was elucidated by spectroscopic analysis (HRMS, 1D and 2D NMR). All the compounds were isolated from F. fimbriata for the first time except for compounds 5 and 12, and compounds 1, 3, 4, 8, 10, 11 and 13 were obtained from this genus for the first time. Compounds 1-4 showed moderate cytotoxic activity against HepG2 cells.
[Studies on phenanthrene constituents from stems of Dendrobium candidum].[Pubmed:19504966]
Zhong Yao Cai. 2009 Feb;32(2):220-3.
OBJECTIVE: To investigate chemical constituents from the stems of Dendrobium candidum. METHODS: The constituents were isolated by various column chromatography methods, and their structures were elucidated by spectral analysis (IR, UV, NMR and MS). RESULTS: Six compounds were isolated from ethanol extract and their structures were identified as 2,3,4,7-tetramethoxyphenanthrene (I), nakaharain (II), 2,5-dihydroxy-3,4-dimethoxyphenanthrene (III), confusarin (IV), Nudol (V) and bulbophyllanthrin (VI). CONCLUSION: Among these compounds, compounds II, III, VI are isolated from Dendrobium candidum for the first time.
Cytotoxic phenanthrenes from the rhizomes of Tamus communis.[Pubmed:16783700]
Planta Med. 2006 Jun;72(8):767-70.
From the fresh rhizomes of Tamus communis five phenanthrenes (1 - 5) were isolated under the guidance of cytotoxic assays in HeLa cells. The compounds were obtained from the highly active CHCl (3) fraction of the MeOH extract by using multistep chromatographic purifications, including VLC, preparative TLC, HPLC and gel filtration. The compounds were identified by means of EI-mass, UV and NMR spectroscopy as 7-hydroxy-2,3,4-trimethoxyphenanthrene (1), 2,7-dihydroxy-3,4-dimethoxyphenanthrene (Nudol) (2), 2,7-dihydroxy-3,4,8-trimethoxyphenanthrene (3), 3,7-dihydroxy-2,4,8-trimethoxyphenanthrene (confusarin) (4), and 3,7-dihydroxy-2,4-dimethoxyphenanthrene (5). Compound 1 is a new natural product, and 2 - 4 were isolated for the first time from T. communis. In the cytotoxic assays, compounds 1 - 3 and 5 significantly inhibited the growth of HeLa cells (IC (50) = 0.97 - 20.18 microM). Compound 3, with an IC (50) value of 0.97 microM, is of special interest because of its high activity.
Spasmolytic stilbenoids from Maxillaria densa.[Pubmed:15567245]
Fitoterapia. 2004 Dec;75(7-8):690-5.
2,5-dihydroxy-3,4-dimethoxyphenanthrene (1), fimbriol-A (2), Nudol (3), gymnopusin (4) and erianthridin (5) isolated from Maxillaria densa provoked a concentration-dependent inhibition of the spontaneous contractions of the rat ileum with potencies comparable to papaverine. In order to establish the mode of action of stilbenoids 1-5, their effect on the contractions induced by different spasmogens (histamine, barium chloride and L-NAME) was investigated. In general, the results suggested that the relaxant activity of the products does not involve a direct nitrergic or antihistaminergic mode of action or an interference with calcium influx into the smooth muscle cells.


