Lambertic AcidCAS# 55051-96-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55051-96-2 | SDF | Download SDF |
| PubChem ID | 13370049.0 | Appearance | Powder |
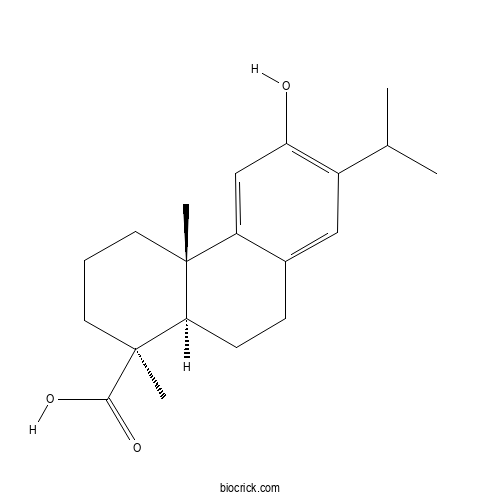
| Formula | C20H28O3 | M.Wt | 316.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 12-Hydroxy-8,11,13-abietatrien-19-oic acid | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (1S,4aS,10aR)-6-hydroxy-1,4a-dimethyl-7-propan-2-yl-2,3,4,9,10,10a-hexahydrophenanthrene-1-carboxylic acid | ||
| SMILES | CC(C)C1=C(C=C2C(=C1)CCC3C2(CCCC3(C)C(=O)O)C)O | ||
| Standard InChIKey | AYDJDNNMKHXZOQ-RLLQIKCJSA-N | ||
| Standard InChI | InChI=1S/C20H28O3/c1-12(2)14-10-13-6-7-17-19(3,15(13)11-16(14)21)8-5-9-20(17,4)18(22)23/h10-12,17,21H,5-9H2,1-4H3,(H,22,23)/t17-,19-,20+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Lambertic Acid Dilution Calculator

Lambertic Acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1602 mL | 15.8008 mL | 31.6016 mL | 63.2031 mL | 79.0039 mL |
| 5 mM | 0.632 mL | 3.1602 mL | 6.3203 mL | 12.6406 mL | 15.8008 mL |
| 10 mM | 0.316 mL | 1.5801 mL | 3.1602 mL | 6.3203 mL | 7.9004 mL |
| 50 mM | 0.0632 mL | 0.316 mL | 0.632 mL | 1.2641 mL | 1.5801 mL |
| 100 mM | 0.0316 mL | 0.158 mL | 0.316 mL | 0.632 mL | 0.79 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ethyl-beta-D- glucoside
Catalog No.:BCX1614
CAS No.:19467-01-7
- Mesatlantin C
Catalog No.:BCX1613
CAS No.:137624-14-7
- Rathbunioside R1
Catalog No.:BCX1612
CAS No.:350689-78-0
- Ophiopogonin A
Catalog No.:BCX1611
CAS No.:11054-24-3
- Spinorhamnoside
Catalog No.:BCX1610
CAS No.:864271-19-2
- 4-Methoxyphenyl-beta-galactoside
Catalog No.:BCX1609
CAS No.:3150-20-7
- Nudol
Catalog No.:BCX1608
CAS No.:86630-46-8
- 3-(4-methoxyphenyl)-3,4-dihydro-2H-chromen-7-ol
Catalog No.:BCX1607
CAS No.:10499-17-9
- 4-(3-oxobutyl)phenyl 6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-beta-D-glucopyranoside
Catalog No.:BCX1606
CAS No.:1609583-04-1
- L-Homoarginine hydrochloride
Catalog No.:BCX1605
CAS No.:1483-01-8
- L-Glutamic acid hydrochloride
Catalog No.:BCX1604
CAS No.:138-15-8
- Orientin-2''-O-p-trans-coumarate
Catalog No.:BCX1603
CAS No.:1229437-75-5
- Eupalinilide A
Catalog No.:BCX1616
CAS No.:757202-06-5
- 3'-Methoxy-5'-hydroxy isoflavone-7-O-beta-D-glucoside
Catalog No.:BCX1617
CAS No.:241129-90-8
- 1,7-dihydroxy-3,4-dimethoxylxanthone-7-O-Veratriloside
Catalog No.:BCX1618
CAS No.:76907-78-3
- 3-O-acetyloleanolicacetic anhydride
Catalog No.:BCX1619
CAS No.:4339-73-5
- Bebeerine
Catalog No.:BCX1620
CAS No.:477-60-1
- Caffeoylmalic Acid
Catalog No.:BCX1621
CAS No.:149197-97-7
- Prostratin
Catalog No.:BCX1622
CAS No.:60857-08-1
- Luteolin 3'-galacturonide
Catalog No.:BCX1623
CAS No.:56317-12-5
- Luteolin 7-galacturonide
Catalog No.:BCX1624
CAS No.:56324-53-9
- 3,4-Dimethoxycinnamic Acid
Catalog No.:BCX1625
CAS No.:14737-89-4
- 10,11-Dimethoxy-17-epi-alpha-yohimbine
Catalog No.:BCX1626
CAS No.:84667-06-1
- N,N'-dimethyldaurisoline iodide
Catalog No.:BCX1627
CAS No.:1422186-34-2
Network Pharmacology and Molecular Docking Reveal the Mechanism of Isodon ternifolius (D. Don) Kudo Against Liver Fibrosis.[Pubmed:37576085]
Drug Des Devel Ther. 2023 Aug 7;17:2335-2351.
AIM: Many studies have demonstrated the hepatoprotective or anti-fibrotic effects of Isodon ternifolius, but its pharmacological basis and mechanism remain unclear. In this study, we used in vitro models to validate the predicted results and revealed the potential mechanism of action and active ingredients through network pharmacology methods and molecular docking. METHODS: The chemical components of Isodon ternifolius were identified by literatures. Potential targets of Isodon ternifolius were predicted by Swiss Target Prediction. The disease targets were collected through the databases of Gene Card. Common targets of Isodon ternifolius and liver fibrosis were obtained by online tool Venny 2.1. PPI protein interaction network was obtained using String database, and target protein interaction network was drawn using Cytoscape software. Signaling pathway enrichment analysis was performed on drug-disease targets with of DAVID database. RESULTS: Twenty-one potential active ingredients and 298 potential targets were predicted by Swiss Target Prediction platform. Ninety pathways related to liver fibrosis were obtained by KEGG enrichment. The TLR4, MAPK and PI3K-Akt signaling pathways are mostly associated with liver fibrosis. Molecular docking techniques were used to validate the core target proteins TNF, Akt1, MAPK1, EGFR and TLR4 binding to the ingredients of Isodon ternifolius, which showed that a multitude of ingredients of Isodon ternifolius were able to bind to the above target proteins, especially 2alpha-hydroxy oleanolic acid and (-)-Lambertic Acid. Our experimental validation results showed that Isodon ternifolius inhibited the activation of PI3K-Akt and ERK1/2 signaling pathways. CONCLUSION: Through a network pharmacology approach and in vitro cell assay, we predicted and validated the active compounds of Isodon ternifolius and its potential targets for LF treatment. The results suggest that the mechanism of Isodon ternifolius treating LF by inhibiting angiogenesis may be related to the ERK1/2 and PI3K/Akt signaling pathways.
Antimicrobial Activities of Salacia oblonga Wall Leaf and Root Extracts Against Different Bacterial Strains and Fungal Isolates.[Pubmed:35612657]
Curr Microbiol. 2022 May 25;79(7):204.
Antibiotic resistance and the hazardous nature of synthetic drugs is threatening issue in the health sector. The alternative for this problem is to focus on plants that attribute to various compounds that exhibit therapeutic properties. Therefore, the study aims to evaluate the antimicrobial efficacy of Salacia oblonga leaf and root extracts against tested human pathogens. The S. oblonga extracts showed a significant zone of inhibition against bacteria and fungi. The leaf and root extracts of S. oblonga are prepared using low polar to high polar solvents in the Soxhlet apparatus and tested on the selected bacterial and fungal strains. Agar well diffusion and broth dilution methods evaluate antibacterial activity, antifungal activity, and Minimum Inhibitory Concentration (MIC) of extracts. Among the extracts tested, the ethyl acetate extract of root showed more antimicrobial activity against the tested bacterial and fungal strains. The most susceptible bacterial and fungal species against ethyl acetate extract are Micrococcus luteus, Mycobacterium tuberculosis, Microsporum canis, Trichophyton interdigitale, and Microsporum gypseum. The MIC for bacteria ranged from 13.0 to > 200 microg/ml, whereas for fungi, the MIC ranged from 25.9 to > 200 microg/ml. Ethyl acetate extract of root with 100 microg/ml concentration showed 29.1 mm and 28.7 mm zone of inhibition against bacterial strains M. luteus and M. tuberculosis, respectively. The ethyl acetate extract of root with a 100 microg/ml concentration showed 15.8, 15.2, and 15.6 mm zone of inhibition against fungal isolates M. canis, T. interdigitale, and M. gypseum, respectively. The activity of root and leaf extracts increased in a concentration-dependent manner, and further, the compounds isolated from the crude extracts of leaf and root showed antimicrobial activity. Structural elucidation of isolated compounds Lambertic Acid and Ferruginol was done using NMR spectroscopy. Reports indicate that Lambertic Acid was isolated previously, but the isolation of hydroxy Ferruginol from S. oblonga leaf extract was reported unprecedented.
Identification and characterization of potential antioxidant components in Isodon amethystoides (Benth.) Hara tea leaves by UPLC-LTQ-Orbitrap-MS.[Pubmed:33385513]
Food Chem Toxicol. 2021 Feb;148:111961.
Isodon amethystoides (Benth.) Hara (IA) tea is a commonly used dietetic Chinese herb and employed for the treatments of tumor and lung abscess. To assess chemical composition and antioxidant capacity of IA leaves extract, a UPLC-LTQ-Orbitrap-MS method and antioxidant tests were used, respectively. 17 compounds were identified including Vinyl caffeate (1), 3,4-dimethoxyphenyl-beta-D-glucopyranoside (2), Rutin (3), Quercetin (4), Loliolide (5), Caffeic acid (6), Rubesanolide D (7), Isorhamnetin (8), Lambertic Acid (9), 6, 7-Dehydroroyleanone (10), Dihydrorabdokunmin C (11), Nervosin (12), Quercitrin (13), Vitexin (14), beta-sitosterol (15), Wangzaozin A (16), Amethystonoic acid (17). Among these, 1-14 compounds were novel and have not been reported ever before in IA while component 10 was a novel finding within this genus. Flavonoid components showed better free radical scavenging ability and profound correlation was observed between diterpenoid compounds content and flavonoids activity. Our results provide experimental basis for extraction and separation of chemical constituents of IA which are antioxidant in nature.


