ProstratinCAS# 60857-08-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60857-08-1 | SDF | Download SDF |
| PubChem ID | 146159333.0 | Appearance | Powder |
| Formula | C22H30O6 | M.Wt | 390.48 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
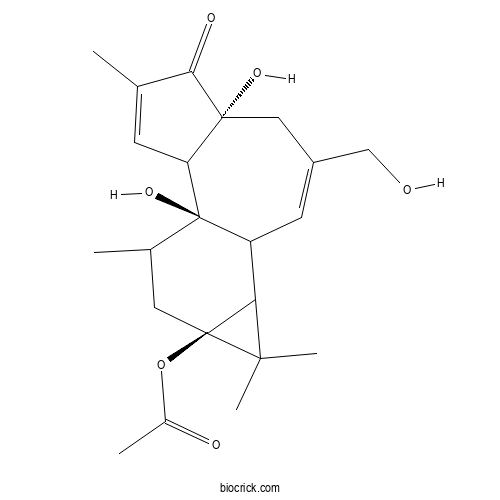
| Chemical Name | [(1R,6R,13S)-1,6-dihydroxy-8-(hydroxymethyl)-4,12,12,15-tetramethyl-5-oxo-13-tetracyclo[8.5.0.02,6.011,13]pentadeca-3,8-dienyl] acetate | ||
| SMILES | CC1CC2(C(C2(C)C)C3C1(C4C=C(C(=O)C4(CC(=C3)CO)O)C)O)OC(=O)C | ||
| Standard InChIKey | BOJKFRKNLSCGHY-IBEWQMDZSA-N | ||
| Standard InChI | InChI=1S/C22H30O6/c1-11-6-16-20(26,18(11)25)9-14(10-23)7-15-17-19(4,5)21(17,28-13(3)24)8-12(2)22(15,16)27/h6-7,12,15-17,23,26-27H,8-10H2,1-5H3/t12?,15?,16?,17?,20-,21+,22-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Prostratin Dilution Calculator

Prostratin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.561 mL | 12.8048 mL | 25.6095 mL | 51.219 mL | 64.0238 mL |
| 5 mM | 0.5122 mL | 2.561 mL | 5.1219 mL | 10.2438 mL | 12.8048 mL |
| 10 mM | 0.2561 mL | 1.2805 mL | 2.561 mL | 5.1219 mL | 6.4024 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5122 mL | 1.0244 mL | 1.2805 mL |
| 100 mM | 0.0256 mL | 0.128 mL | 0.2561 mL | 0.5122 mL | 0.6402 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Caffeoylmalic Acid
Catalog No.:BCX1621
CAS No.:149197-97-7
- Bebeerine
Catalog No.:BCX1620
CAS No.:477-60-1
- 3-O-acetyloleanolicacetic anhydride
Catalog No.:BCX1619
CAS No.:4339-73-5
- 1,7-dihydroxy-3,4-dimethoxylxanthone-7-O-Veratriloside
Catalog No.:BCX1618
CAS No.:76907-78-3
- 3'-Methoxy-5'-hydroxy isoflavone-7-O-beta-D-glucoside
Catalog No.:BCX1617
CAS No.:241129-90-8
- Eupalinilide A
Catalog No.:BCX1616
CAS No.:757202-06-5
- Lambertic Acid
Catalog No.:BCX1615
CAS No.:55051-96-2
- Ethyl-beta-D- glucoside
Catalog No.:BCX1614
CAS No.:19467-01-7
- Mesatlantin C
Catalog No.:BCX1613
CAS No.:137624-14-7
- Rathbunioside R1
Catalog No.:BCX1612
CAS No.:350689-78-0
- Ophiopogonin A
Catalog No.:BCX1611
CAS No.:11054-24-3
- Spinorhamnoside
Catalog No.:BCX1610
CAS No.:864271-19-2
- Luteolin 3'-galacturonide
Catalog No.:BCX1623
CAS No.:56317-12-5
- Luteolin 7-galacturonide
Catalog No.:BCX1624
CAS No.:56324-53-9
- 3,4-Dimethoxycinnamic Acid
Catalog No.:BCX1625
CAS No.:14737-89-4
- 10,11-Dimethoxy-17-epi-alpha-yohimbine
Catalog No.:BCX1626
CAS No.:84667-06-1
- N,N'-dimethyldaurisoline iodide
Catalog No.:BCX1627
CAS No.:1422186-34-2
- α-Cannabispiranol
Catalog No.:BCX1628
CAS No.:69636-83-5
- 4-Hydroxyderricin
Catalog No.:BCX1629
CAS No.:55912-03-3
- (2β,3β,4α,16α)-2,3,16,23-Tetrahydroxyolean-13(18)-en-28-oic acid
Catalog No.:BCX1630
CAS No.:2226630-77-7
- 12β-Acetoxyganoderic Acid θ
Catalog No.:BCX1631
CAS No.:2374206-90-1
- 2-[3-(3,7-Dimethyl-2,6-octadien-1-yl)-2,4-dihydroxyphenyl]-5,7-dihydroxy-4H-1-benzopyran-4-one
Catalog No.:BCX1632
CAS No.:2226467-12-3
- (7aR,8R,13aR,13bS,13cR)-Dodecahydro-8-hydroxy-1H,5H,10H-dipyrido[2,1-f:3',2',1'-ij][1,6]naphthyridin...
Catalog No.:BCX1633
CAS No.:2306139-04-6
- Epiberberine chloride
Catalog No.:BCX1634
CAS No.:889665-86-5
Hub Genes, Possible Pathways and Predicted Drugs in Hereditary Gingival Fibromatosis by Bioinformatics Analysis.[Pubmed:38546525]
Chin J Dent Res. 2024 Mar 28;27(1):101-109.
OBJECTIVE: To explore potential pathogenic processes and possible treatments using unbiased and reliable bioinformatic tools. METHODS: Gene expression profiles of control and hepatocyte growth factor (HGF) samples were downloaded from CNP0000995. Analysis of differentially expressed genes (DEGs) was conducted using R software (version 4.2.1, R Foundation, Vienna, Austria). Functional enrichment analyses were performed using the Gene Ontology (GO), Kyoto Encyclopaedia of Genes and Genomes (KEGG) and Gene Set Enrichment Analysis (GSEA) databases, then the proteinprotein interaction (PPI) network was constructed to screen the top 10 hub genes. Finally, five genes related to cell junctions were selected to build gene-miRNA interactions and predict small-molecule drugs. RESULTS: A total of 342 downregulated genes and 188 upregulated genes were detected. Candidate pathways include the extracellular matrix (ECM) receptor interaction pathway, the TGF-beta signalling pathway and the cell adhesion molecule (CAM) pathway, which were discovered through KEGG and GSEA enrichment studies. GO analyses revealed that these DEGs were significantly enriched in cell adhesion, the adherens junction and focal adhesion. Five hub genes (CDH1, SNAP25, RAC2, APOE and ITGB4) associated with cell adhesion were identified through PPI analysis. Finally, the gene-miRNA regulatory network identified three target miRNAs: hsa-miR-7110-5p, hsa-miR-149-3p and hsa-miR-1207-5p. Based on the gene expression profile, the small-molecule drugs zebularine, ecuronium and Prostratin were selected for their demonstrated binding activity when docked with the mentioned molecules. CONCLUSION: This study offered some novel insights into molecular pathways and identified five hub genes associated with cell adhesion. Based on these hub genes, three potential therapeutic miRNAs and small-molecule drugs were predicted, which are expected to provide guidance for the treatment of patients with HGF.
Total Syntheses of Phorbol and 11 Tigliane Diterpenoids and Their Evaluation as HIV Latency-Reversing Agents.[Pubmed:38486375]
J Am Chem Soc. 2024 Mar 27;146(12):8746-8756.
Tigliane diterpenoids possess exceptionally complex structures comprising common 5/7/6/3-membered ABCD-rings and disparate oxygen functionalities. While tiglianes display a wide range of biological activities, compounds with HIV latency-reversing activity can eliminate viral reservoirs, thereby serving as promising leads for new anti-HIV agents. Herein, we report collective total syntheses of phorbol (13) and 11 tiglianes 14-24 with various acylation patterns and oxidation states, and their evaluation as HIV latency-reversing agents. The syntheses were strategically divided into five stages to increase the structural complexity. First, our previously established sequence enabled the expeditious preparation of ABC-tricycle 9 in 15 steps. Second, hydroxylation of 9 and ring-contractive D-ring formation furnished phorbol (13). Third, site-selective attachment of two acyl groups to 13 produced four phorbol diesters 14-17. Fourth, the oxygen functionalities were regio- and stereoselectively installed to yield five tiglianes 18-22. Fifth, further oxidation to the most densely oxygenated acerifolin A (23) and tigilanol tiglate (24) was realized through organizing a 3D shape of the B-ring. Assessment of the HIV latency-reversing activities of the 12 tiglianes revealed seven tiglianes (14-17 and 22-24) with 20- to 300-fold improved efficacy compared with Prostratin (12), a representative latency-reversing agent. Therefore, the robust synthetic routes to a variety of tiglianes with promising activities devised in this study provide opportunities for advancing HIV eradication strategies.
Novel Triazolopyridine-Based BRD4 Inhibitors as Potent HIV-1 Latency Reversing Agents.[Pubmed:38229757]
ACS Med Chem Lett. 2023 Dec 14;15(1):60-68.
Bromodomain-containing protein 4 (BRD4) inhibitors have been proven to be a promising option for anti-HIV-1 latency therapeutics. We herein describe the design, synthesis, and anti-HIV-1 latency bioevaluation of triazolopyridine derivatives as BRD4 inhibitors. Among them, compound 13d displayed favorable HIV-1 reactivation and prominent safety profile without triggering abnormal immune activation. It exerted strong synergism when combined with the PKC activator Prostratin and has the same BRD4-targeting latency mechanism as observed with JQ1, by stimulating Tat-dependent HIV-1 elongation. Besides, it neither affected the antiviral efficacies of antiviral drugs nor caused secondary infections to uninfected cells and the latency reversing potency of 13d, in turn, was not affected by different classes of antiviral drugs.
Terpenoids from the Roots of Stellera chamaejasme (L.) and Their Bioactivities.[Pubmed:38067457]
Molecules. 2023 Nov 23;28(23):7726.
An undescribed diterpene, stellerterpenoid A (1), and two undescribed sesquiterpenoids, stellerterpenoids B and C (2-3), together with six known compounds, Prostratin (4) stelleraguaianone B (5), chamaejasnoid A (6), auranticanol L (7), wikstronone C (8), and oleodaphnone (9), were isolated from the roots of Stellera chamaejasme L. Their structures were elucidated by extensive spectroscopic data (1D, 2D NMR, IR, UV, and HR-ESI-MS). The absolute configuration of 1-3 was elucidated based on ECD calculation. Among them, stellerterpenoid A was a rare 13, 14-seco nortigliane diterpenoid and stellerterpenoid B was a guaiacane-type sesquiterpenoid with an unusual 1, 2-diketone moiety. The known stelleraguaianone B (5) exhibited moderate activity for suppressing NO production in lipopolysaccharide (LPS)-treated RAW 264.7 macrophages cells with an IC(50) value of 24.76 +/- 0.4 muM. None of the compounds showed anti-influenza virus or anti-tumor activity in vitro.
Automated microarray platform for single-cell sorting and collection of lymphocytes following HIV reactivation.[Pubmed:37693052]
Bioeng Transl Med. 2023 Jun 21;8(5):e10551.
A promising strategy to cure HIV-infected individuals is to use latency reversing agents (LRAs) to reactivate latent viruses, followed by host clearance of infected reservoir cells. However, reactivation of latent proviruses within infected cells is heterogeneous and often incomplete. This fact limits strategies to cure HIV which may require complete elimination of viable virus from all cellular reservoirs. For this reason, understanding the mechanism(s) of reactivation of HIV within cellular reservoirs is critical to achieve therapeutic success. Methodologies enabling temporal tracking of single cells as they reactivate followed by sorting and molecular analysis of those cells are urgently needed. To this end, microraft arrays were adapted to image T-lymphocytes expressing mCherry under the control of the HIV long terminal repeat (LTR) promoter, in response to the application of LRAs (Prostratin, iBET151, and SAHA). In response to Prostratin, iBET151, and SAHA, 30.5%, 11.2%, and 12.1% percentage of cells, respectively. The arrays enabled large numbers of single cells (>25,000) to be imaged over time. mCherry fluorescence quantification identified cell subpopulations with differing reactivation kinetics. Significant heterogeneity was observed at the single-cell level between different LRAs in terms of time to reactivation, rate of mCherry fluorescence increase upon reactivation, and peak fluorescence attained. In response to Prostratin, subpopulations of T lymphocytes with slow and fast reactivation kinetics were identified. Single T-lymphocytes that were either fast or slow reactivators were sorted, and single-cell RNA-sequencing was performed. Different genes associated with inflammation, immune activation, and cellular and viral transcription factors were found.
Scopoletin Reactivates Latent HIV-1 by Inducing NF-kappaB Expression without Global T Cell Activation.[Pubmed:37628826]
Int J Mol Sci. 2023 Aug 10;24(16):12649.
Reversing HIV-1 latency promotes the killing of infected cells and is essential for cure strategies. However, current latency-reversing agents (LRAs) are not entirely effective and safe in activating latent viruses in patients. In this study, we investigated whether Scopoletin (6-Methoxy-7-hydroxycoumarin), an important coumarin phytoalexin found in plants with multiple pharmacological activities, can reactivate HIV-1 latency and elucidated its underlying mechanism. Using the Jurkat T cell model of HIV-1 latency, we found that Scopoletin can reactivate latent HIV-1 replication with a similar potency to Prostratin and did so in a dose- and time-dependent manner. Moreover, we provide evidence indicating that Scopoletin-induced HIV-1 reactivation involves the nuclear factor kappa B (NF-kappaB) signaling pathway. Importantly, Scopoletin did not have a stimulatory effect on T lymphocyte receptors or HIV-1 receptors. In conclusion, our study suggests that Scopoletin has the potential to reactivate latent HIV-1 without causing global T-cell activation, making it a promising treatment option for anti-HIV-1 latency strategies.
Esterification with a Long-Chain Fatty Acid Elevates the Exposure Toxicity of Tigliane Diterpenoids from Euphorbia fischeriana Roots against Nematodes.[Pubmed:37599642]
J Agric Food Chem. 2023 Aug 30;71(34):12730-12740.
In this study, two tigliane diterpenoids, 12-deoxyphorbol-13-hexadecanoate and 12-deoxyphorbol-13-acetate (Prostratin), were identified from the methanol extract of the roots of Euphorbia fischeriana and were found to have the ability to significantly reduce the survival of Caenorhabditis elegans. It was determined that exposure to these two compounds had toxic effects on the growth, reproduction, locomotion behavior, and accumulation of lipids and lipofuscin of the nematodes. Moreover, the transcription levels of the genes associated with lipid accumulation, apoptosis, insulin, and nuclear hormone synthesis in C. elegans were significantly influenced. Interestingly, 12-deoxyphorbol-13-hexadecanoate produced exposure toxicity at lower concentrations than that of Prostratin. Pearson correlation analysis indicates that the elevated exposure toxicity of 12-deoxyphorbol-13-hexadecanoate may be the result of differing transcription levels, which result from the differential expression of fat-6, egl-38, and cep-1. These results reveal that esterification with a long-chain fatty acid elevates the exposure toxicity of this tigliane diterpenoid, thus providing a basis for the application of tigliane diterpenoids in plant-derived nematicides.
Genetic variation of the HIV-1 subtype C transmitted/founder viruses long terminal repeat elements and the impact on transcription activation potential and clinical disease outcomes.[Pubmed:37307292]
PLoS Pathog. 2023 Jun 12;19(6):e1011194.
A genetic bottleneck is a hallmark of HIV-1 transmission such that only very few viral strains, termed transmitted/founder (T/F) variants establish infection in a newly infected host. Phenotypic characteristics of these variants may determine the subsequent course of disease. The HIV-1 5' long terminal repeat (LTR) promoter drives viral gene transcription and is genetically identical to the 3' LTR. We hypothesized that HIV-1 subtype C (HIV-1C) T/F virus LTR genetic variation is a determinant of transcriptional activation potential and clinical disease outcome. The 3'LTR was amplified from plasma samples of 41 study participants acutely infected with HIV-1C (Fiebig stages I and V/VI). Paired longitudinal samples were also available at one year post-infection for 31 of the 41 participants. 3' LTR amplicons were cloned into a pGL3-basic luciferase expression vector, and transfected alone or together with Transactivator of transcription (tat) into Jurkat cells in the absence or presence of cell activators (TNF-alpha, PMA, Prostratin and SAHA). Inter-patient T/F LTR sequence diversity was 5.7% (Renge: 2-12) with subsequent intrahost viral evolution observed in 48.4% of the participants analyzed at 12 months post-infection. T/F LTR variants exhibited differential basal transcriptional activity, with significantly higher Tat-mediated transcriptional activity compared to basal (p<0.001). Basal and Tat-mediated T/F LTR transcriptional activity showed significant positive correlation with contemporaneous viral loads and negative correlation with CD4 T cell counts (p<0.05) during acute infection respectively. Furthermore, Tat-mediated T/F LTR transcriptional activity significanly correlated positively with viral load set point and viral load; and negatively with CD4 T cell counts at one year post infection (all p<0.05). Lastly, PMA, Prostratin, TNF-alpha and SAHA cell stimulation resulted in enhanced yet heterologous transcriptional activation of different T/F LTR variants. Our data suggest that T/F LTR variants may influence viral transcriptional activity, disease outcomes and sensitivity to cell activation, with potential implications for therapeutic interventions.
NSC95397 is a Novel HIV Latency Reversing Agent.[Pubmed:37293110]
bioRxiv [Preprint]. 2023 May 25:2023.05.24.542213.
The latent viral reservoir represents one of the major barriers of curing HIV. Focus on the "kick and kill" approach, in which virus expression is reactivated then cells producing virus are selectively depleted, has led to the discovery of many latency reversing agents (LRAs) that can reactivate latently integrated virus and further our understanding of the mechanisms driving HIV latency and latency reversal. Thus far, individual compounds have yet to be robust enough to work as a therapy, highlighting the importance of identifying new compounds that can act in novel pathways and synergize with known LRAs. In this study, we identified a promising LRA, NSC95397, from a screen of ~4250 compounds in J-Lat cell lines. We validated that NSC95397 reactivates latent viral transcription and protein expression from cells with unique integration events. Cotreating cells with NSC95397 and known LRAs demonstrated that NSC95397 has the potential to synergize with different drugs, such Prostratin, a PKC agonist, and SAHA, an HDAC inhibitor. By looking at multiple common markers of open chromatin, we show that NSC95397 does not increase open chromatin globally. Bulk RNA sequencing revealed that NSC95397 does not greatly change cellular transcription. Instead, NSC95397 downregulates many pathways key to metabolism, cell growth, and DNA repair - highlighting the potential of these pathways in regulating HIV latency. Overall, we identified NSC95397 as a novel LRA that does not alter global transcription, that shows potential for synergy with known LRAs, and that may act through novel pathways not previously recognized for their ability to modulate HIV latency.
Discovering the Mechanisms of Oleodaphnone as a Potential HIV Latency-Reversing Agent by Transcriptome Profiling.[Pubmed:37108519]
Int J Mol Sci. 2023 Apr 16;24(8):7357.
Latent HIV is a key factor that makes AIDS difficult to cure. Highly effective and specific latent HIV activators can effectively activate latent HIV, and then combined with antiretroviral therapy to achieve a functional cure of AIDS. Here, four sesquiterpenes (1-4) including a new one (1), five flavonoids (5-9) including three biflavonoid structures, and two lignans (10 and 11) were obtained from the roots of Wikstroemia chamaedaphne. Their structures were elucidated through comprehensive spectroscopic analyses. The absolute configuration of 1 was determined by experimental electronic circular dichroism. NH2 cell model was used to test the activity of these 11 compounds in activating latent HIV. Oleodaphnone (2) showed the latent HIV activation effect as well as the positive drug Prostratin, and the activation effect was time- and concentration-dependent. Based on transcriptome analysis, the underlying mechanism was that oleodaphnone regulated the TNF, C-type lectin receptor, NF-kappaB, IL-17, MAPK, NOD-like receptor, JAK-Stat, FoxO, and Toll-like receptor signaling pathways. This study provides the basis for the potential development of oleodaphnone as an effective HIV latency-reversing agent.
An overview of the traditional use, phytochemistry, and biological activity of the genus Homalanthus.[Pubmed:36871869]
Fitoterapia. 2023 Apr;166:105466.
Homalanthus species are native to tropical Asia and the Pacific region. This genus, comprising 23 accepted species, received less scientific attention compared to other genera of the Euphorbiaceae family. Seven Homalanthus species, such as H. giganteus, H. macradenius, H. nutans, H. nervosus, N. novoguineensis, H. populneus, and H. populifolius, have been reported to treat various health problems in traditional medicine. Only a few Homalanthus species have been investigated for their biological activities, including antibacterial, anti-HIV, anti-protozoal, estrogenic, and wound-healing activities. From a phytochemical point of view ent-atisane, ent-kaurane, and tigliane diterpenoids, triterpenoids, coumarins, and flavonol glycosides were found to be characteristic metabolites of the genus. The most promising compound is Prostratin, isolated from H. nutans, with anti-HIV activity and the ability to eradicate the HIV reservoir in infected patients by mechanism of protein kinase C (PKC) agonist. This review provides information on traditional usage, phytochemistry, and biological activity of the genus Homalanthus with the aim to delineate future research directions.
Automated microarray for single-cell sorting and collection of lymphocytes following HIV reactivation.[Pubmed:36778314]
bioRxiv [Preprint]. 2023 Feb 3:2023.02.02.526757.
A promising strategy to cure HIV infected individuals is to use latency reversing agents (LRAs) to reactivate latent viruses, followed by host clearance of infected reservoir cells. However, reactivation of latent proviruses within infected cells is heterogeneous and often incomplete. This fact limits strategies to cure HIV which may require complete elimination of viable virus from all cellular reservoirs. For this reason, understanding the mechanism(s) of reactivation of HIV within cellular reservoirs is critical to achieve therapeutic success. Methodologies enabling temporal tracking of single cells as they reactivate followed by sorting and molecular analysis of those cells are urgently needed. To this end, microraft arrays were adapted to image T-lymphocytes expressing mCherry under the control of the HIV long terminal repeat (LTR) promoter, in response to the application of various LRAs (Prostratin, iBET151, and SAHA). In response to Prostratin, iBET151, and SAHA, 30.5 %, 11.2 %, and 12.1 % percentage of cells respectively, reactivated similar to that observed in other experimental systems. The arrays enabled large numbers of single cells (>25,000) to be imaged over time. mCherry fluorescence quantification identified cell subpopulations with differing reactivation kinetics. Significant heterogeneity was observed at the single cell level between different LRAs in terms of time to reactivation, rate of mCherry fluorescence increase upon reactivation, and peak fluorescence attained. In response to Prostratin, subpopulations of T lymphocytes with slow and fast reactivation kinetics were identified. Single T-lymphocytes that were either fast or slow reactivators were sorted, and single-cell RNA-sequencing was performed. Different genes associated with inflammation, immune activation, and cellular and viral transcription factors were found. These results advance our conceptual understanding of HIV reactivation dynamics at the single-cell level toward a cure for HIV.
Natural killer cells induce HIV-1 latency reversal after treatment with pan-caspase inhibitors.[Pubmed:36561752]
Front Immunol. 2022 Dec 6;13:1067767.
The establishment of a latency reservoir is the major obstacle for a cure of HIV-1. The shock-and-kill strategy aims to reactivate HIV-1 replication in HIV -1 latently infected cells, exposing the HIV-1-infected cells to cytotoxic lymphocytes. However, none of the latency reversal agents (LRAs) tested so far have shown the desired effect in people living with HIV-1. We observed that NK cells stimulated with a pan-caspase inhibitor induced latency reversal in co-cultures with HIV-1 latently infected cells. Synergy in HIV-1 reactivation was observed with LRAs Prostratin and JQ1. The supernatants of the pan-caspase inhibitor-treated NK cells activated the HIV-1 LTR promoter, indicating that a secreted factor by NK cells was responsible for the HIV-1 reactivation. Assessing changes in the secreted cytokine profile of pan-caspase inhibitor-treated NK cells revealed increased levels of the HIV-1 suppressor chemokines MIP1alpha (CCL3), MIP1beta (CCL4) and RANTES (CCL5). However, these cytokines individually or together did not induce LTR promoter activation, suggesting that CCL3-5 were not responsible for the observed HIV-1 reactivation. The cytokine profile did indicate that pan-caspase inhibitors induce NK cell activation. Altogether, our approach might be-in combination with other shock-and-kill strategies or LRAs-a strategy for reducing viral latency reservoirs and a step forward towards eradication of functionally active HIV-1 in infected individuals.
4-phenylquinoline-8-amine induces HIV-1 reactivation and apoptosis in latently HIV-1 infected cells.[Pubmed:36527748]
Biochem Biophys Res Commun. 2023 Jan 22;641:139-147.
Combinational antiretroviral therapy (cART) dramatically suppresses the viral load to undetectable levels in human immunodeficiency virus (HIV)-infected patients. However, HIV-1 reservoirs in CD4+T cells and myeloid cells, which can evade cART and host antiviral immune systems, are still significant obstacles to HIV-1 eradication. The "Shock and Kill" approach using latently-reversing agents (LRAs) is therefore currently developing strategies for effective HIV-1 reactivation from latency and inducing cell death. Here, we performed small-molecular chemical library screening with monocytic HIV-1 latently-infected model cells, THP-1 Nluc #225, and identified 4-phenylquinoline-8-amine (PQA) as a novel LRA candidate. PQA induced efficient HIV-1 reactivation in combination with PKC agonists including Prostratin and showed a similar tendency for HIV-1 activation in primary HIV-1 reservoirs. Furthermore, PQA induced killing of HIV-1 latently-infected cells. RNA-sequencing analysis revealed PQA had different functional mechanisms from PKC agonists, and oxidative stress-inducible genes including DDIT3 or CTSD were only involved in PQA-mediated cell death. In summary, PQA is a potential LRA lead compound that exerts novel functions related to HIV-1 activation and apoptosis-mediated cell death to eliminate HIV-1 reservoirs.
Impact of IL-15 and latency reversing agent combinations in the reactivation and NK cell-mediated suppression of the HIV reservoir.[Pubmed:36329160]
Sci Rep. 2022 Nov 3;12(1):18567.
Inhibitors of histone deacetylases (HDACis) are major latency reversing agent (LRA) candidates in 'shock and kill' strategies to eradicate the HIV reservoir in infected patients. The poor achievements of initial HDACi-based trials and subsequent studies have highlighted the need for more efficient approaches such as combinatory and immunostimulating therapies. Here we studied combinations of IL-15 with pan-HDACi (Vorinostat, Romidepsin, Panobinostat) or class I selective-HDACi (Entinostat) with or without a PKC agonist (Prostratin) for their impact on in vitro reactivation and NK cell-mediated suppression of latent HIV. Results showed that pan-HDACis but not Entinostat reduced NK cell viability and function; yet, combined IL-15 reverted the negative effects of pan-HDACis except for Panobinostat. All HDACis were ineffective at reactivating HIV in a CD4(+) T cell model of latency, with pan-HDACis suppressing spontaneous and IL-15- or Prostratin-induced HIV release, while IL-15 + Prostratin combination showed maximal activity. Moreover, Panobinostat impaired STAT5 and NF-kappaB activation by IL-15 and Prostratin, respectively. Finally, by using effectors (NK) and targets (latently infected CD4(+) T cells) equally exposed to drug combinations, we found that IL-15-mediated suppression of HIV reactivation by NK cells was inhibited by Panobinostat. Our data raise concerns and encouragements for therapeutic application of IL-15/LRA combinations.


