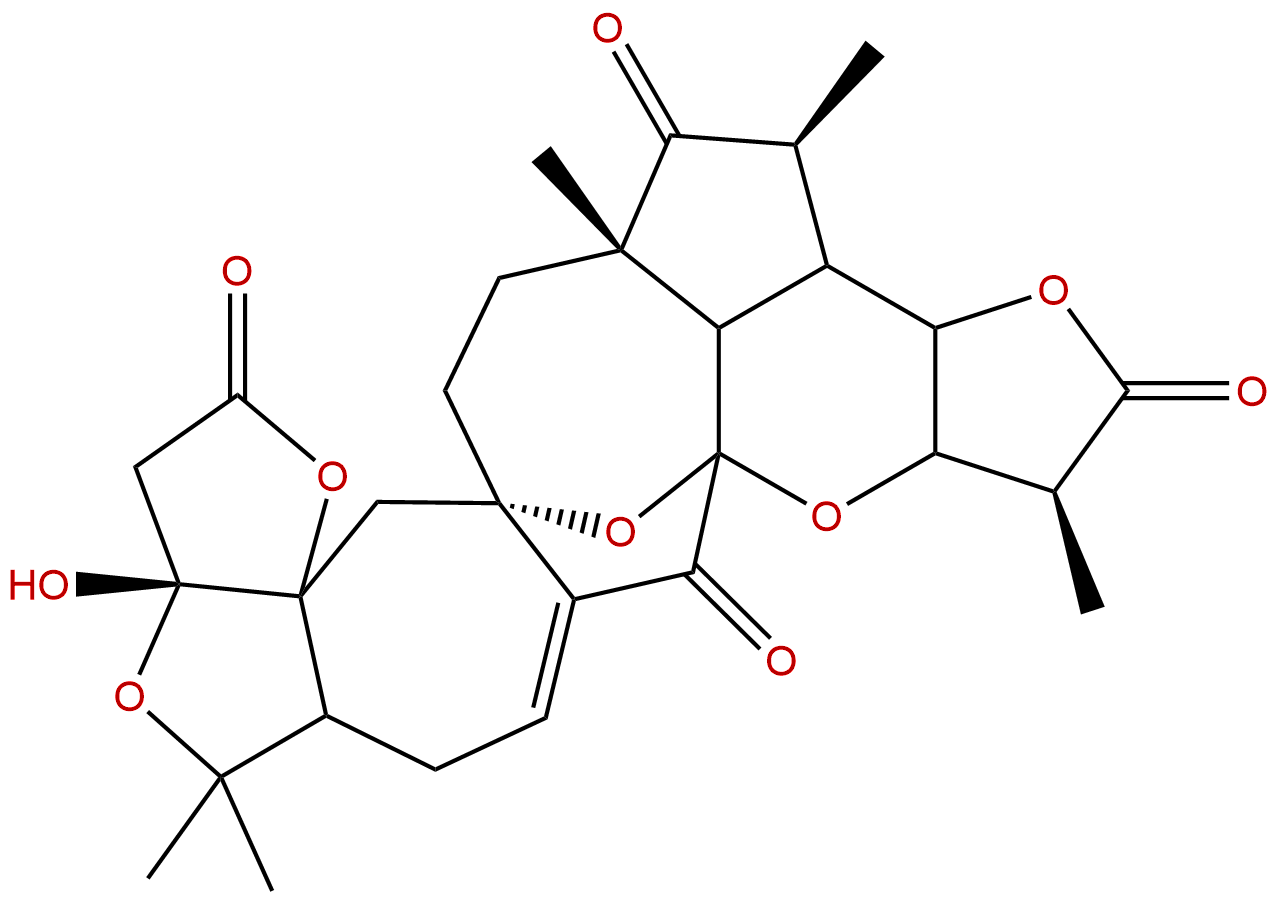
Schindilactone ACAS# 943239-45-0 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 943239-45-0 | SDF | Download SDF |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C29H34O10 | M.Wt | 542.58 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Schindilactone A Dilution Calculator

Schindilactone A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.843 mL | 9.2152 mL | 18.4305 mL | 36.8609 mL | 46.0762 mL |
| 5 mM | 0.3686 mL | 1.843 mL | 3.6861 mL | 7.3722 mL | 9.2152 mL |
| 10 mM | 0.1843 mL | 0.9215 mL | 1.843 mL | 3.6861 mL | 4.6076 mL |
| 50 mM | 0.0369 mL | 0.1843 mL | 0.3686 mL | 0.7372 mL | 0.9215 mL |
| 100 mM | 0.0184 mL | 0.0922 mL | 0.1843 mL | 0.3686 mL | 0.4608 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Apigenin-7-O-glucuronide-6'-ethyl ester
Catalog No.:BCX1598
CAS No.:62268-42-2
- 14-Formyldihydrorutaecarpine
Catalog No.:BCX1597
CAS No.:68353-23-1
- Arisanlactone D
Catalog No.:BCX1596
CAS No.:1234186-01-6
- Oleaside B
Catalog No.:BCX1595
CAS No.:71699-08-6
- Vinaginsenoside R7
Catalog No.:BCX1594
CAS No.:156009-83-5
- Telephiose C
Catalog No.:BCX1593
CAS No.:297749-31-6
- Daphneticin
Catalog No.:BCX1592
CAS No.:83327-22-4
- Wuweizidilactone A
Catalog No.:BCX1591
CAS No.:945610-99-1
- Telephiose A
Catalog No.:BCX1590
CAS No.:297749-29-2
- HuangjiangSu A
Catalog No.:BCX1589
CAS No.:1026020-27-8
- Thalictricoside
Catalog No.:BCX1588
CAS No.:649758-25-8
- Trikamsteroside A
Catalog No.:BCX1587
CAS No.:942916-76-9
- Isobergaptol
Catalog No.:BCX1600
CAS No.:21339-45-7
- Uvarimacrophin A
Catalog No.:BCX1601
CAS No.:167425-75-4
- L-Cysteine hydrochloride monohydrate
Catalog No.:BCX1602
CAS No.:7048-04-6
- Orientin-2''-O-p-trans-coumarate
Catalog No.:BCX1603
CAS No.:1229437-75-5
- L-Glutamic acid hydrochloride
Catalog No.:BCX1604
CAS No.:138-15-8
- L-Homoarginine hydrochloride
Catalog No.:BCX1605
CAS No.:1483-01-8
- 4-(3-oxobutyl)phenyl 6-O-[(2E)-3-(4-hydroxyphenyl)prop-2-enoyl]-beta-D-glucopyranoside
Catalog No.:BCX1606
CAS No.:1609583-04-1
- 3-(4-methoxyphenyl)-3,4-dihydro-2H-chromen-7-ol
Catalog No.:BCX1607
CAS No.:10499-17-9
- Nudol
Catalog No.:BCX1608
CAS No.:86630-46-8
- 4-Methoxyphenyl-beta-galactoside
Catalog No.:BCX1609
CAS No.:3150-20-7
- Spinorhamnoside
Catalog No.:BCX1610
CAS No.:864271-19-2
- Ophiopogonin A
Catalog No.:BCX1611
CAS No.:11054-24-3
Asymmetric Total Syntheses of Hypoestin A, Albolic Acid, and Ceroplastol II.[Pubmed:35657330]
J Am Chem Soc. 2022 Jun 15;144(23):10162-10167.
The first asymmetric total synthesis of bioactive diterpenoid hypoestin A with an unprecedented [5-8-5-3] tetracyclic skeleton is accomplished in 15 steps from commercially available (R)-limonene. Furthermore, the second asymmetric total syntheses of sesterterpenoids albolic acid and ceroplastol II in 21 steps are also reported. The synthetically challenging and highly functionalized [X-8-5] (X = 5 or 7) tricarbocyclic ring systems found in hypoestin A, albolic acid, ceroplastol II, and Schindilactone A, as well as other natural products, are efficiently and directly constructed via a unique intramolecular Pauson-Khand reaction of an allene-yne. This work represents the first reported use of the Pauson-Khand reaction to access synthetically challenging eight-membered-ring systems in natural product synthesis.
The Journey of Schinortriterpenoid Total Syntheses.[Pubmed:30681828]
Acc Chem Res. 2019 Feb 19;52(2):480-491.
Plants in the Schisandraceae family are important components of the traditional Chinese herbal medicines and are often used to treat various illnesses. Therefore, these Schisandraceae plants are valuable sources for the discovery of new chemical entities for novel therapeutic development. Considerable progress has been made in the identification of bioactive and structurally novel triterpenoids from the Schisandraceae family in the past two decades. In particular, Sun and co-workers have successfully isolated over 100 nortriterpenoids from the Schisandraceae family. Some of these nortriterpenoids have strong inhibitory activities toward hepatitis, tumors, and HIV-1. However, the natural scarcity of these nortriterpenoids in the Schisandraceae plants has hampered their isolation and further biomedical development, and their biosynthesis has not been fully elucidated. It is therefore important and urgent to develop efficient and streamlined total syntheses of these medicinally important nortriterpenoids. Such syntheses will provide sufficient materials for detailed biological studies as well as new synthetic analogues and probe molecules to improve their biological functions and elucidate their mode of actions. However, because of their structural novelty and complexity, the total syntheses of these nortriterpenoid natural products present a significant challenge for synthetic chemists, despite the progress made in organic synthesis, particularly total synthesis, in the 20th century and since the beginning of the 21st century. New synthetic methodologies and strategies therefore need to be invented and developed to facilitate the total syntheses of these nortriterpenoid natural products. With this in mind, our group has spent the last 15 years, ever since the isolation of micrandilactone A (1) by Sun and co-workers in 2003 ( Sun et al. Org. Lett. 2003 , 5 , 1023 - 1026 ), working on synthetic studies with a view to developing methods and strategies for the total syntheses of schinortriterpenoids. Enabling methods such as a thiourea/Pd-catalyzed alkocycarbonylative annulation and a thiourea/Co-catalyzed Pauson-Khand reaction have been developed under these circumstances to form the key ring systems and stereocenters of these complex target molecules. These methodological advances have led us to the first total syntheses of Schindilactone A (2), lancifodilactone G acetate (6a), 19-dehydroxyarisandilactone A (9), and propindilactone G (10) with diverse structural features via a branching-oriented strategy. The chemistry developed during our total synthesis campaign has not only helped us to deal with various challenges encountered in the syntheses of the four target molecules, but has also opened up new avenues for synthesizing other naturally occurring schinortriterpenoids and their derivatives, which will likely result in molecules with improved biological functions and tool compounds to enable elucidation of their mechanism of actions or potential cellular targets. This Account highlights the chemistry evolution of our schinortriterpenoid syntheses.
Diastereoselective total synthesis of (+/-)-schindilactone a, Part 3: The final phase and completion.[Pubmed:22761030]
Chem Asian J. 2012 Oct;7(10):2341-50.
The final phase for the total synthesis of (+/-)-Schindilactone A (1) is described herein. Two independent synthetic approaches were developed that featured Pd-thiourea-catalyzed cascade carbonylative annulation reactions to construct intermediate 3 and a RCM reaction to make intermediate 4. Other important steps that enabled the completion of the synthesis included: 1) A Ag-mediated ring-expansion reaction to form vinyl bromide 17 from dibromocyclopropane 30; 2) a Pd-catalyzed coupling reaction of vinyl bromide 17 with a copper enolate to synthesize ketoester 16; 3) a RCM reaction to generate oxabicyclononenol 10 from diene 11; 4) a cyclopentenone fragment in substrate 8 was constructed through a Co-thiourea-catalyzed Pauson-Khand reaction (PKR); 5) a Dieckmann-type condensation to successfully form the A ring of Schindilactone A (1). The chemistry developed for the total synthesis of Schindilactone A (1) will shed light on the synthesis of other family members of Schindilactone A.
Diastereoselective total synthesis of (+/-)-schindilactone A, Part 2: Construction of the fully functionalized CDEFGH ring system.[Pubmed:22761018]
Chem Asian J. 2012 Oct;7(10):2334-40.
The successful synthesis of the highly complex model compound (2) of the CEFGH ring system of Schindilactone A (1) is described. Several synthetic methodologies were developed and applied to achieve this goal, including ring-closing metathesis (RCM) and Co-thiourea-catalyzed Pauson-Khand reactions. Furthermore, two independent approaches were developed for the construction of the GH ring of model compound 2, the key steps of which included Pd-thiourea-catalyzed carbonylative annulation, methylation, and sequential RCM/oxa-Michael-addition reactions. The chemistry developed herein has provided a greater understanding of the synthesis of Schindilactone A (1) and its analogous compounds of the same family.
Diastereoselective total synthesis of (+/-)-schindilactone A, Part 1: Construction of the ABC and FGH ring systems and initial attempts to construct the CDEF ring system.[Pubmed:22761005]
Chem Asian J. 2012 Oct;7(10):2321-33.
First-generation synthetic strategies for the diastereoselective total synthesis of Schindilactone A (1) are presented and methods for the synthesis of the ABC, FGH, and CDEF moieties are explored. We have established a method for the synthesis of the ABC moiety, which included both a Diels-Alder reaction and a ring-closing metathesis as the key steps. We have also developed a method for the synthesis of the FGH moiety, which involved the use of a Pauson-Khand reaction and a carbonylative annulation reaction as the key steps. Furthermore, we have achieved the construction of the central 7-8 bicyclic ring system by using a [3,3]-rearrangement as the key step. However, unfortunately, when this rearrangement reaction was applied to the construction of the more advanced CDEF moiety, the anticipated annulation reaction did not occur and the development of an alternative synthetic strategy would be required for the construction of this central core.


